Update on denosumab and SRE’s: data from the 147 trial
A little over ten years ago, I lost my Father to metastatic prostate cancer, a brave fight that lasted only a few years since he was diagnosed with stage IV disease. In many ways though, it was a bittersweet moment because while it’s always heartbreaking to lose a parent, it was also a blessing that he was spared of any further pain. In those days, the only bisphosphonates available in the UK were pamidronate (Aredia) and the awful clodronate. The urologist managing my Father had no clue about either, so he suffered in silence instead.
I tell this story because, while difficult to discuss, sometimes we forget the importance of supportive care and pain management in improving the quality of life for patients with advanced cancer.

Based on my family’s experience, I can tell you that it makes a huge difference, not just to the patient, but also for other family members. There is nothing more distressing to see someone in considerable pain from bone destruction and the doctors not doing anything about it because ‘it’s normal for the condition’ or they are considered ‘too old over 65’. In the end, I suspect many give up the fight because the pain becomes unbearable.
Since my Father’s passing, clinical advances in managing skeletal related events (SREs) and bone pain have thankfully moved on with the approval of another more potent bisphosphonate, zoledronic acid (Zometa) and a RANK-L inhibitor, denosumab (Xgeva). Both of these therapies increase the time to SREs associated with metastases compared to placebo, but what about bone metastases and survival – do they make any difference?
New clinical data now available
This morning at the AUA annual meeting, data was presented by Dr Matthew Smith (Mass General) on the much awaited update on the 147 phase III trial in the late breaking plenary session. The study compared denosumab (120 mg subcutaneously taken monthly) to placebo.
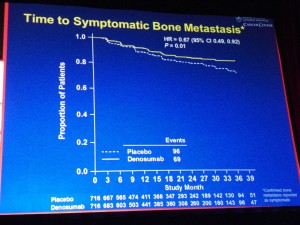
Overall, in terms of efficacy, denosumab significantly:
– Increased bone metastasis-free survival by a median of 4.2 months compared with placebo ie 29.5 and 25.2 months, respectively (hazard ratio [HR] 0.85; 95% CI: 0.73, 0.98; P=0.028; risk reduction of 15%)
– Delayed time to first bone metastasis compared with placebo (HR 0.84; 95% CI: 0.71, 0.98; P=0.032; risk reduction of 16%)
– Delayed time to symptomatic bone metastasis (HR 0.67; 95% CI: 0.49, 0.92; P=0.01).
However, it wasn’t all good news on the efficacy front though.
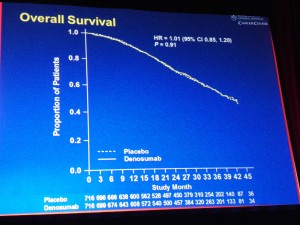
Median overall survival was similar between groups (HR 1.01; 95% CI: 0.85, 1.20; P=0.91).
In other words, patients did not live longer when given denosumab therapy compared to placebo.
Comparison of side effects
Overall, rates of adverse events (AEs) and serious AEs were generally similar between groups, with two exceptions, namely:
– Yearly cumulative incidence of osteonecrosis of jaw was similar to rates previously reported for monthly denosumab 120 mg (year 1: 1.1%, year 2: 2.9%, year 3: 4.2%), although the overall cumulative rate was 4.6% (n=33).
– Hypocalcemia occurred in 1.7% (n=12) denosumab and 0.3% (n=2) placebo patients.
To put this in context, in the zoledronic acid versus placebo trial, both ONJ and hypocalcemia were lower than that seen in the denosumab study. According to Medscape Reference, this is not a side effect to be trifled with, since it can cause stroke and cardiopulmonary effects:
“Depending on the cause, unrecognized or poorly treated hypocalcemic emergencies can lead to significant morbidity or death.”
Clearly, some of the effects can be mitigated with calcium and vitamin D supplementation, although some patients, especially though with co-morbidities, may be challenging with adherence and compliance.
A critical question is are surrogate measurements clinically meaningful?
Dr Oliver Sartor raised this issue recently in a Nature publication, essentially challenging whether SRE was clinically meaningful or not, and arguing that better measurements would be a reduction in pain or improvement in the patient’s quality life when considering whether a therapy had an impact or not.
You can find out more about Dr Sartor’s insights in a recent blog post my colleague, Pieter Droppert, posted on Biotech Strategy Blog in the run-up to the AUA meeting.
In order to get to the bottom of this dilemma, I interviewed Dr Neal Shore, a urologist at the Carolina Urologic Research Center, and asked him what he thought of the significance of the data:
“This is a huge milestone in our understanding of the bone microenvironment”
On managing the adverse events versus the improved time to bone-free metastasis, Dr Shore replied,
“I think the risk benefit of preventing a skeletal metastasis versus the small, but albeit statistically real risk of ONJ, far outweighs that risk.”
The interview with Dr Shore will post tomorrow morning as a podcast as part of the Ongoing Making a Difference series – do check back to hear his insights on the 147 trial.
References:
![]() Sartor, O. (2011). Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life Asian Journal of Andrology DOI: 10.1038/aja.2011.33
Sartor, O. (2011). Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life Asian Journal of Andrology DOI: 10.1038/aja.2011.33
7 Responses to “Update on denosumab and SRE’s: data from the 147 trial”
Hi I can see that in essence we are delaying ‘pain’ here by 4 months.
But 4% of patients are developing ONJ with 2% Hypercalcaemia.
If you throw in the $20K plus treatment cost is there still a clear cost / benefit profile here?
thanks
Km
Good question. I think the cost-benefit trade-off ultimately comes down to whether you have insurance coverage or not. If you have none, then your perception of the cost of initial treatment and managing the potential side effects with be very different than if you have a 20% co-pay and the insurance company pays the rest.
The risk of ONJ and hypocalcemia are relatively small at less than 5% and in an interview with Dr Shore he did mention that prophylaxis and conservative management helped to minimise them. He had some thoughts to share on this issue, so I will clean up the recording and post it soon.
[…] Biotech Strategy Blog ← Update on denosumab and SRE’s: data from the 147 trial […]
[…] none of the other therapies targeting bone in prostate cancer such as zoledronic acid (Zometa), denosumab (Xgeva) or cabozantinib (XL184) have shown any overall survival […]
[…] out from other drugs that are targeting bone metastases in CRPC, such as cabozantinib (XL184) and denosumab (Xgeva®), where to my knowledge no overall survival benefits have yet been […]
[…] It will be interesting to see whether cabozantinib can impact overall survival (OS) in advanced prostate cancer, something that denosumab (Xgeva®) failed to show in the 147 trial that was just presented at AUA. […]
[…] Church on Pharma Strategy Blog wrote about the denosumab 147 data presented at the annual meeting of the American Urological Association (AUA 2011) last […]
Comments are closed.