Update on PARP inhibitors including iniparib, olaparib and veliparib
A PSB reader wrote in asking whether an update on the PARP inhibitors and the clinical trials would be possible. Following on from the last update in January that covered Sanofi’s negative iniparib phase III data in triple negative breast cancer and AstraZeneca’s decision in February not to pursue olaparib in hereditary BRCA1 and 2 positive breast cancers, it would be a good idea to see what’s left of this once highly promising class of compounds.
I first wrote about PARP inhibitors way back in 2006 and like many, I’m rather disappointed with the results we’ve seen so far. However, all is not lost. Abbott’s veliparib is going strong, while Pfizer (PF-01367338) and Cephalon (CEP-9722) are just getting started with their programs.
Iniparib was probably the weakest inhibitor of the class and perhaps not potent enough, since there was no increase in toxicities in the TNBC study (that can be a good and a bad thing), while olaparib has proven to be potent but challenging to combine with chemotherapy. It doesn’t mean that a different compound or clinical approach will be unsuccessful.
The saddest thing about the iniparib trial is the lack of BRCA1 and 2 testing, given the heterogeneous nature of triple negative breast cancer. We will likely never know which different subsets responded and why from that trial, it probably could have been better designed and included more rigorous biopsies for biomarker analysis, but once done it is too late. This is one of the dangers of applying old-style chemotherapy trial designs to targeted therapies – first know your molecular targets – or potential targets – and evaluate the biomarkers over time in response to therapy. Otherwise, it’s a bit like blindfolding an archer and asking him to hit a target s/he can’t even see.
I don’t think all is lost with AstraZeneca’s olaparib yet, but we will have to wait and see what the current ongoing studies bring in terms of answers. Certainly, both AstraZeneca and Abbott have a broad range of clinical trials that may yield some interesting results. We shall see.
I took a quick look at the clinical trials database and sifted through the available data for PARP inhibitors. This is what we have so far:
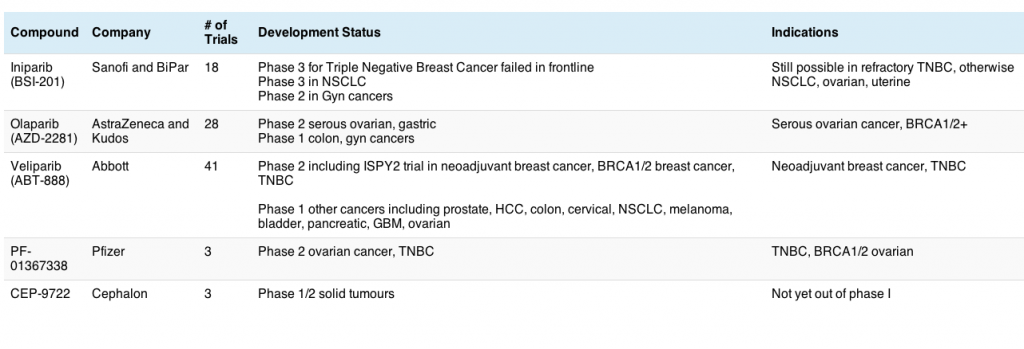
One trial I’m eagerly awaiting the results of is the ISPY2 trial in neoadjuvant breast cancer, which included veliparib as one of the treatment options in a molecular based approac,h much in the same way the BATTLE trial worked in lung cancer. For those interested in the background to this approach in breast cancer, you can find the details in an interview with Sue Desmond-Hellmann (UCSF), when the trial was first announced. It will be a while before we know the results, but one that is very eagerly awaited in the breast cancer community.
11 Responses to “Update on PARP inhibitors including iniparib, olaparib and veliparib”
That is correct way to explain this information.. Thanks for announcing this..
Regarding BRCA testing, are there legal issues with Myriad’s patent on the BRCA genes that is discouraging Sanofi and other pharmas from prospectively testing for mutations in their clinical trial design?
That is possible, but I think it more likely that Sanofi wanted as broad an indication possible. Limiting it to BRCA1/2 in triple negative breast cancer would have reduced the commercial opportunity considerably.
This really nice information and great way of explanation.
a recent ASCO abstract sheds some light on the lack of activity for iniparib (BSI-201), confirming an oft heard rumor that iniparib isn’t really a PARP inhibitor http://abstract.asco.org/AbstView_102_79153.html
Hello everyone, I,m looking for help to my younger sister, amaizing young women- she has tragic situation – over one Year is fighting with breast cancer triple negative, (without BRCA1,2) verry rare, after surgery and a lot of chemotherapy before and after but it doesn’t work, she has a lot of metastaticks to bones, but she is still fighting, it is second cancer in her life already, 10 yeas ago she win with but now the situation is very critical, together with har doctor we had a lots of hope connecting with PARP inhibitors, this can be har only chance, maybe someone can help how to get the possibility to attend in such clinical trials with this drugs, please look at her profile, maybe You can help, she still can do many good thing in her life: http://www.goldenline.pl/marta-biedron
Anna, I’m very sorry indeed to hear about your sister.
The best way to get access to the PARP inhibitors is to contact her oncologist and discuss whether there are either any clinical trials available in your area, or failing that, the oncologist can ask the companies (sanofi, AstraZeneca, Abbott or Pfizer) for compassionate use.
Anna,
contact the NCI (National Cancer institue) in Bethesda, Maryland. They have clincial trials with PARP inhibitors and they help with finances even if you have health insurance. They are covering my airfare to/from Alaska and helping me with room and board.
Hello, how about Parp BSI-201 for Glioblastoma ? Any good news ?
Thanks Maria
Thank you very much~~
M12-895 study is currently conducted at Stanford Cancer Center. I don’t think that they indicated in the clinical trial table above. This is phase II study of Veliparib in combination with TMZ or in combination with Carboplatin and Paclitaxel versus placebo in subjects with BRCA1 or BRCA 2 mutation and metastatic breast cancer.
Comments are closed.