We all know the importance that inflammation and the immune system plays in the early development of many cancers, but this is not a ubiquitous finding. Indeed, some hematologic malignancies arise out of immunodeficiency, such as myelomas and chronic lymphocytic leukemia (CLL). I haven’t written much about this topic in the past, so thought it would be useful to explain some of the underlying biology of CLL given that we can expect new (hopefully positive) data emerge at the American Society of Clinical Oncology (ASCO) meeting in June.
Several studies have shown specifically that immune activation can promote development and progression of lymphoma. Extranodal marginal zone lymphomas (eMZLs), for example, originate at the site of chronic inflammation under the influence of T-cell help (see Suarez et al., 2006).
Hervé et al., (2005) have previously shown that antigen receptor repertoires exist in B-cell lymphoma and additionally, autoantigen recognition by CLL-derived immunoglobulin may suggest a role for antigen receptor signaling in lymphomagenesis.
In addition, an increase in circulating regulatory T cells (Tregs) has been reported in myeloma, CLL, and other B-cell lymphomas by Breyer et al., (2005) and confirmed by others. This may well explain why the combination of fludarabine and cyclophosphamide (FC) is a useful strategy in reducing immunosuppression prior to cancer immunotherapy with rituximab (R). The FCR combination is now very much the bedrock of treatment for patients with CLL and is based on a very solid rationale.
Previous work in 2004 by Christopoulos et al., observed a significant reduction in peripheral T helper cells in patients with untreated FL and eMZL. The question remains as to what happens to the T-helper cells in untreated CD4+ subpopulations.
Christopoulos et al., (2011) therefore conducted a prospective open label study recently to look at the underlying immune system in patients with CLL or monoclonal gammopathy of unknown significance (MGUS). According to the Mayo Clinic:
“Monoclonal gammopathy of undetermined significance (MGUS) is a condition in which an abnormal protein (monoclonal protein, or M protein) is in the blood.”
In terms of the selection criteria, patients with prior antineoplastic therapy, including corticosteroids, and patients with evidence for preexisting autoimmunity or immunodeficiency were excluded from the study.
The current study is interesting, because the results demonstrated:
“substantially reduced circulating T helper cells, predominantly naive CD4+ cells, in patients with nonleukemic follicular lymphoma and extranodal marginal zone lymphoma, but not in monoclonal gammopathy and early CLL.”
They went to to say that:
“Gene expression profiling of in vitro–stimulated CD4+ cells revealed an independent second alteration of T helper cell physiology, which was most pronounced in early CLL but also detectable in follicular lymphoma/extranodal marginal zone lymphoma.”
What new therapies are emerging for CLL?
Rituximab has been a very useful addition to the basic FC regimen in CLL. A number of other therapies are also being evaluated in the disease, including fostamatinib, a SYK inhibitor from AstraZeneca and lenalidomide, a widely used immunotherapy for the treatment of multiple myeloma from Celgene.
Friedberg et al., (2010) published their data on fostamatinib in CLL and NHL last year based on the promising results previously presented at ASCO in 2009. I wrote about the data at that meeting here for those interested. Lenalidomide is being evaluated as a maintenance therapy for CLL in clinical trials (see my notes on the previous data presented at ASH) and given it is now 18 months to two years since we saw the initial data, I’m hoping there will be an update in Chicago this year, including some information on the optimal lenalidomide dose (see Wendtner et al., 2010).
Although CLL is a relatively indolent disease, patients can cycle through multiple therapies and combinations over time, so there is still a need for new drugs to mix up the combinations and extend life for this chronic condition further.
A future update will appear on this topic at ASCO in June – watch this space!
References:
 Christopoulos, P., Pfeifer, D., Bartholome, K., Follo, M., Timmer, J., Fisch, P., & Veelken, H. (2011). Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL Blood, 117 (14), 3836-3846 DOI: 10.1182/blood-2010-07-299321
Christopoulos, P., Pfeifer, D., Bartholome, K., Follo, M., Timmer, J., Fisch, P., & Veelken, H. (2011). Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL Blood, 117 (14), 3836-3846 DOI: 10.1182/blood-2010-07-299321
Suarez F, Lortholary O, Hermine O, & Lecuit M (2006). Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood, 107 (8), 3034-44 PMID: 16397126
Hervé M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, Chiorazzi N, & Meffre E (2005). Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. The Journal of clinical investigation, 115 (6), 1636-43 PMID: 15902303
Christopoulos P, Follo M, Fisch P, & Veelken H (2008). The peripheral helper T-cell repertoire in untreated indolent B-cell lymphomas: evidence for antigen-driven lymphomagenesis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K, 22 (10), 1952-4 PMID: 18385751
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, & Shipp MA (2010). Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood, 115 (13), 2578-85 PMID: 19965662
Wendtner CM (2011). Lenalidomide in CLL: What Is the Optimal Dose? Clinical advances in hematology & oncology : H&O, 9 (3), 220-4 PMID: 21475128
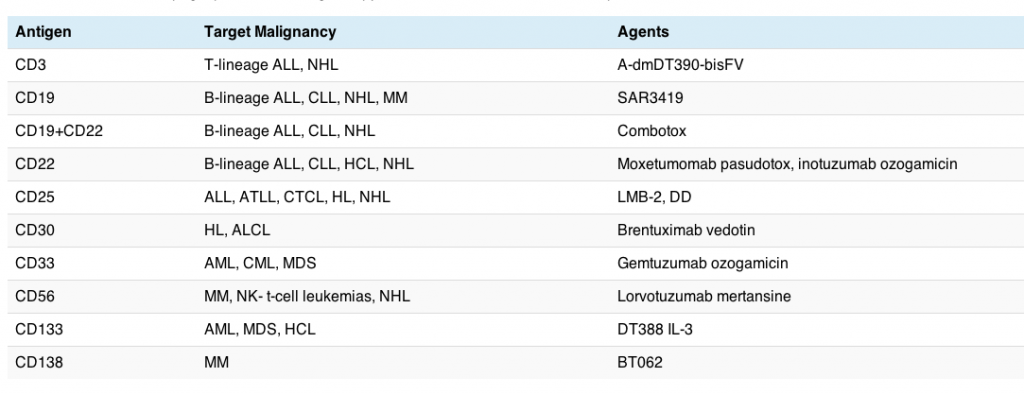
![]() FitzGerald, D., Wayne, A., Kreitman, R., & Pastan, I. (2011). Treatment of Hematologic Malignancies with Immunotoxins and Antibody-Drug Conjugates Cancer Research, 71 (20), 6300-6309 DOI: 10.1158/0008-5472.CAN-11-1374
FitzGerald, D., Wayne, A., Kreitman, R., & Pastan, I. (2011). Treatment of Hematologic Malignancies with Immunotoxins and Antibody-Drug Conjugates Cancer Research, 71 (20), 6300-6309 DOI: 10.1158/0008-5472.CAN-11-1374
