Update on pancreatic Neuroendocrine Tumours (NET)
Today on 10th November, it’s the Second Annual Worldwide NET (Neuroendocrine Tumor) Cancer Awareness Day. Granted that’s a bit of a mouthful, but it also seems poignant given so many of my news feeds this morning were still full of Steve Jobs, who sadly passed away from the disease last month.
I’ve been meaning to post an update on this rare form of cancer all year, given that we now have targeted therapies now approved by the FDA for treatment, but things were hectic at the office and then with Jobs passing, the timing just seemed tacky and inappropriate.
The idea though, of an Awareness Day for a rare disease such as NET to improve both education and awareness seems an inherently good one to me, especially as there has been some progress clinically in 2011. According to Kulke et al., (2011) NET has an incidence of around 1 per 100,000 individuals. This excellent review covers the key essentials of both the disease and the treatments:
“Patients with pancreatic NET present with diverse symp- toms related to hormonal hypersecretion, tumor bulk, or both. Accurate diagnosis of this condition and differentiation of pancreatic NET from the more common pancreatic adenocarcinomas is a critical first step in developing an appropriate treatment plan.”
It has been quite the landmark year for NET since not one, but two, new therapies were approved by the FDA in May this year with very different mechanisms of action:
- Everolimus (Afinitor) from Novartis targets the mammalian target of rapamycin (mTOR), a serine-threonine kinase, downstream of the PI3K/AKT pathway.
- Sunitinib (Sutent) from Pfizer is a multikinase inhibitor of VEGF, PDGFR (α and β), KIT, FLT3, RET and CSF-1R.
Both of these drugs are also approved for the treatment of renal cell cancer, which while nearby geographically in the body, is a completely different type of GI cancer. They are now approved for pancreatic neuroendocrine tumours (pNET) that have progressed and cannot be treated with surgery.
It is important to note, that while NET is a rare form of pancreatic cancer, it is not the same thing as the more common pancreatic adenocarcinoma – a fact that the media often got wrong in the case of Steve Jobs and drove me potty at their ignorance and inability to grasp a simple concept. NET is not an adenocarcinoma and has a much larger endocrinology/metabolism component and starts in the hormone-producing cells of the pancreas. There are two types of pancreatic NET:
- Functional (overproduce hormones)
- Nonfunctional (do not overproduce hormones) and are more common
There are some great resources for patients (and caregivers) want to know more about this disease – here are some examples I came across:
- The Pancreatic NET Connections site sponsored by Pfizer gives some great education about the disease
- The NET Alliance site is sponsored by Novartis and offers lots of useful information
- Pancreatic Cancer Action Network has a nifty Facebook page
- The American Cancer Society has information about treatments
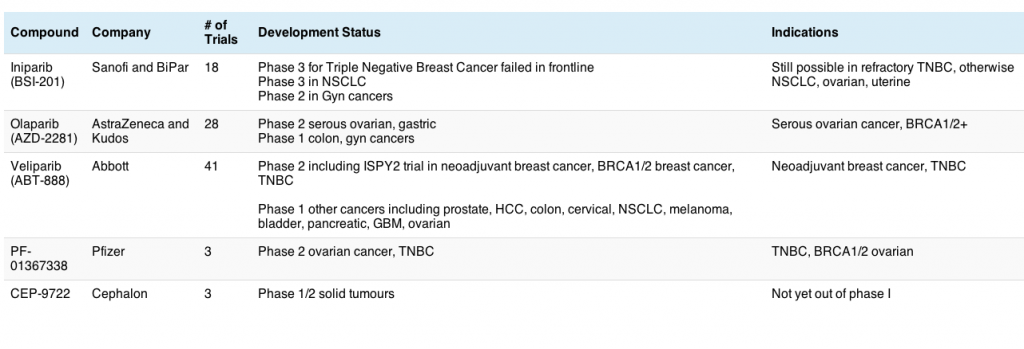
Let’s take a look at the new clinical data. Both sunitinib and everolimus were compared to placebo in the phase III trial of refractory patients who were ineligible for surgery and had disease progression.
Here’s what the survival curves look like, based on the data from their respective prescribing information: 12
In the case of sunitinib, we can see that the median progression-free survival (PFS) 10.2 months versus 5.4 months for the placebo arm. This difference was highly significant (P 0.000146, HR 0.427):

Looking at the everolimus data, we also see a significant trend in favour of therapy over placebo, i.e. a median PFS of 11.04 months compared with 4.6 months for placebo (P 0.001, HR 0.35):

These studies produced very comparable responses from a survival perspective and overall response rates of 9% and 5%, respectively.
While it is good to see some excellent progress on the efficacy front, these new therapies for NET are not without their challenges and side effects though.
In particular, in the phase III study, sunitinib, hypertension was the most common grade 3 event in 10% of patients (a classic function of the VEGF class of drugs) and was also shown to cause cardiac failure leading to death in 2/83 (2%) patients on therapy compared with no patients on placebo.
In contrast, everolimus should be avoided with concomitant use of strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole). The most common grade 3/4 adverse reactions (incidence ≥ 5%) in the phase III pNET trial were stomatitis and diarrhea.
As Kulke et al., observed:
“Surgical resection remains the mainstay of treatment for patients with localized disease.”
However, for patients who are unresectable or have progressed there are at last new options:
“Recent studies have also reported that the tyrosine kinase inhibitor sunitinib and the mTOR inhibitor everolimus improved progression-free survival in patients with pancreatic NET, further expanding the therapeutic arsenal available to patients with this disease.”
In the future, we may well see sequencing studies emerge as well as other targeted therapies to prolong outcomes for patients with this rare disease.
References:
![]() Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, & Yao J (2011). Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. Journal of hematology & oncology, 4 PMID: 21672194
Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, & Yao J (2011). Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. Journal of hematology & oncology, 4 PMID: 21672194
 According to the
According to the