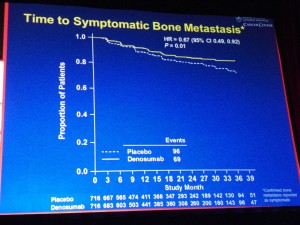
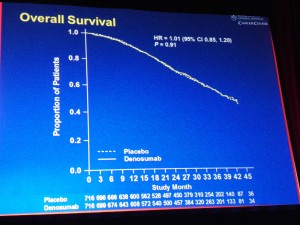
Prostate cancer is very much in the news this morning, not all for good reasons though.
Dendreon’s Provenge launch to community oncologists did not go well
Dendreon’s stock is in free fall after the company missed it’s earnings and revenue expectations rather badly yesterday. Adam Feuerstein of The Street has a nice overview of the 2Q earnings call for those of you interested.
There are a couple of things that come to mind though:
- The reimbursement may well have a broader impact on the landscape than many realise – CMS may pay for a drug or vaccine, but it doesn’t always pay for the surrounding expenses associated with it*
- The “cost dense” issue is offset by ipilimumab (Yervoy) doing better than expected in their metastatic melanoma launch despite a higher overall price (execution matters!)
- The root of Dendreon’s problem may well be lack of demand and healthy scepticism from medical oncologists over the value of Provenge relative to the cost:benefit (no impact on tumour shrinkage, bone pain, etc; patients just live a little longer)
- Strategy and execution are key in cancer launches to community oncologists
* There are some excellent reimbursement experts out there such as my good friend Bobbi Buell at Covad who steer companies through this kind of minefield. In fair disclosure, I know I appreciated and valued her solid advice when I was at Novartis and we launched Gleevec in CML. Having such expertise is a necessity, not a luxury, these days.
Medivation’s MDV3100 may have new opportunities
There was good news from Medivation that caught my interest. Medivation are developing their androgen receptor (AR) antagonist, MDV3100, in castrate resistant prostate cancer pre and post chemotherapy, with interim results from the latter possibly expected by the year end. Today, the company announced some positive preclinical data in breast cancer:
“Researchers at the University of Colorado Denver… provide evidence that MDV3100 inhibits proliferation of breast cancer cells.
In different cell-based assays, MDV3100 was able to inhibit both androgen- and estrogen-mediated breast cancer cell proliferation.”
What are the significance of these findings? Well, the company quoted one of the study authors, Dr Jennifer Richter:
“Our findings are exciting because they challenge the existing idea that androgens are protective in breast cancer by demonstrating that androgens can stimulate proliferation of breast cancer.
These preclinical data show that MDV3100 suppresses androgen-driven breast cancer cell growth and, surprisingly, also suppresses estrogen-driven breast cancer cell growth.”
I think it would be reasonable to expect a phase I clinical trial to evolve soon to test the hypothesis in women with hormone-sensitive breast cancer.
TMPRSS2:ERG may be a more useful marker than PSA in prostate cancer
The big news that really cheered me most this morning, though, was new data from Science Translational Medicine showing the feasibility of a simple urine test to pick up signs of prostate cancer potentially earlier than we do now. Having had a father who was suddenly diagnosed with stage IV disease, hearing about a test that may help diagnose it earlier than currently feasible with biopsies or PSA is a most welcome advance.
Back in 2005, Arul Chinnaiyan’s lab reported a fusion between two genes present in around half of all prostate cancers called TMPRSS2:ERG. When the two genes, TMPRSS2 and ERG, combine, they cause aberrant activity and drive prostate cells to grow out of control, leading to cancer. This is in much in the same way BCR-ABL drives aberrant activity in chronic myeloid leukemia (CML). The next step after the discovery was to evaluate the reliability and faithfulness of the gene in indicating whether men had prostate cancer.
In the latest report, the researchers measured the level of the fused gene in the urine of men (n=1312) with high PSA levels in their blood, then looked at TMPRSS2:ERG levels, tumour volume and clinically significant prostate cancer, and PCA levels. They analysed the data to see if the two markers were a good indication of prostate cancer or not.
Half of the men sampled were found to have the TMPRSS2:ERG gene, confirming previous research by the group.
The results also demonstrated:
“TMPRSS2:ERG, in combination with urine prostate cancer antigen 3 (PCA3), improved the performance of the multivariate Prostate Cancer Prevention Trial risk calculator in predicting cancer on biopsy.”
Essentially, this means that by combining the TMPRSS2:ERG results with PCA data, the group have found a way to stratify men with prostate cancer in terms of risk – in other words, they have a larger and more invasive tumour that requires aggressive treatment.
The diagnostic technology was developed by Gen-Probe, so I think it would be reasonable to assume the company will submit the data to the FDA for approval once further tests have been completed to evaluate accuracy and specificity of the test. If those are successful, we may well have a new diagnostic test for prostate cancer in the not too distant future.
It goes without saying that picking up aggressive disease earlier and treating it effectively will likely lead to better outcomes for men with prostate cancer.
References:
 Tomlins, S., Aubin, S., Siddiqui, J., Lonigro, R., Sefton-Miller, L., Miick, S., Williamsen, S., Hodge, P., Meinke, J., Blase, A., Penabella, Y., Day, J., Varambally, R., Han, B., Wood, D., Wang, L., Sanda, M., Rubin, M., Rhodes, D., Hollenbeck, B., Sakamoto, K., Silberstein, J., Fradet, Y., Amberson, J., Meyers, S., Palanisamy, N., Rittenhouse, H., Wei, J., Groskopf, J., & Chinnaiyan, A. (2011). Urine TMPRSS2:ERG Fusion Transcript Stratifies Prostate Cancer Risk in Men with Elevated Serum PSA Science Translational Medicine, 3 (94), 94-94 DOI: 10.1126/scitranslmed.3001970
Tomlins, S., Aubin, S., Siddiqui, J., Lonigro, R., Sefton-Miller, L., Miick, S., Williamsen, S., Hodge, P., Meinke, J., Blase, A., Penabella, Y., Day, J., Varambally, R., Han, B., Wood, D., Wang, L., Sanda, M., Rubin, M., Rhodes, D., Hollenbeck, B., Sakamoto, K., Silberstein, J., Fradet, Y., Amberson, J., Meyers, S., Palanisamy, N., Rittenhouse, H., Wei, J., Groskopf, J., & Chinnaiyan, A. (2011). Urine TMPRSS2:ERG Fusion Transcript Stratifies Prostate Cancer Risk in Men with Elevated Serum PSA Science Translational Medicine, 3 (94), 94-94 DOI: 10.1126/scitranslmed.3001970