ASH 2011 Update 1: Bruton’s Kinase Inhibitors (BTK)
This year’s American Society of Hematology (ASH) meeting heralded a wealth of new information on pipeline compounds in early development. Although a lot of people were excited about myelofibrosis and the battle between Incyte’s ruxolitinib and YM Bioscience’s CYT387 (more on these in a separate update), the area that intrigued me most was the Bruton’s Tyrosine Kinase (BTK) inhibitors in B-cell lymphomas.
Background on the science behind the BTK pathway:
I’ve been following these novel agents for a while and was fascinated by two abstracts from the ASCO and ASH meetings last year. It became clear that BTK is a valid target in B-cell lymphomas after Advani et al., (2010) demonstrated at ASCO the effect of BTK inhibitor PCI-32765 monotherapy on responses in patients with relapsed aggressive NHL.1
Later that year, Ponader et al., (2010) showed that PCI-32765 abrogates BCR- and nurselike cell-derived activation of CLL cells in vitro and in vivo.2
Between them, these two abstracts offered a solid rationale for investigating BTK inhibitors in B-cell lymphomas and CLL.
Where are we now with clinical development?
Fast forward to ASH this year, Dr Susan O’Brien presented the initial phase I/II data in chronic lymphocytic leukemia (CLL), which was well worth waiting for the last day of the conference to hear. She took us through the concept of the how the BTK pathway fits into B-cell malignancies:
 Source: Dr Susan O’Brien ASH 2011 CLL oral session, reprinted with permission 3
Source: Dr Susan O’Brien ASH 2011 CLL oral session, reprinted with permission 3
Essentially BTK is a critical part of the BCL pathway that leads to proliferation, so targeting it leads to cell death or apoptosis:
“BTK is a Tec family kinase that is required for B-cell activation mediated by BCR signaling. The essential role of BTK in normal B-cell development is evidenced by the clinical syndrome X-linked agammaglobulinemia, in which BCR signaling is abrogated by mutations in BTK.
Signaling from the BCR is also believed to be required for the maintenance of cell division and survival in B cell malignancies, presumably via downstream phosphorylation of PLC-gamma by BTK, ultimately leading to the activation of the anti-apoptotic transcription factor NF-kB and the kinase ERK.
Additionally, BTK may also play a role in the pathogenesis of B-cell malignancies by regulating integrin-mediated migration and adhesion, through regulation of malignant cell response to lymph node-derived chemotactic factors, such as CXCL12 and CXCL13.”
What does the latest data show?
Here’s a quick overview of the preclinical and clinical data I managed to see at ASH. There were over 2,000 posters over three days, plus a day and a half of simultaneous oral sessions, so it was quite hard to keep up with the sheer volume of it all!
In mantle cell lymphoma (MCL) the preclinical data looked encouraging:
Ponader et al., (2011) provided a nice overview of the initial PCI-32765 preclinical data in mantle cell lymphoma (MCL) in a poster presntation.4
They concluded that MCL cells express surface IgM and BTK, which is involved in BCR signalling. In this study, PCI-32765 successfully blocked BTK function and inhibited MCL proliferation, except in resistant cell lines. The former explains why there were responses in patients, while the presence of the latter suggests that additional BTK independent pathways exist and need to be elucidated.
I think that figuring the mechanisms of resistance out is important because it will help suggest possible rational combinations with BTKi therapy in advanced disease.
In CLL, the initial phase I/II clinical data is early, but promising:
Over the last 18 months, patients with CLL (n=117) have been enrolled into five trials, although the interim data was reported in the two relapsed/refractory arms (asterisked arms) at this conference, at two different dose cohorts of PCI-32765, given orally daily at either 420mg (n=27) or 840mg (n=34):

Source: Dr Susan O’Brien ASH 2011 CLL oral session, reprinted with permission5
The overall response rate (ORR) of 67-68% for both doses in the relapsed, refractory setting showed impressive activity and a clear sign that the BTK target is a valid one in this setting. Although the CR rate was low (<5%), the majority of patients saw PRs in this setting. Those with bulky disease (common in advanced disease) tended to do better.
There were also dramatic changes in the tumour burden, with the majority of patients seeing a greater than 50% change from baseline. Sustained improvements in blood counts were also reported. What particularly caught my attention was the activity in patients with known poor risk factors such as 11q and 17p deletions, who tend to have noticeably poorer outcomes. Obviously this is only a small phase I/II trial and we will have to see what happens in a larger scale randomised phase III study to see if the results are reproducible.
Typical side effects were diarrhea, cough and fatigue (any grade and grade 2+). The most common grade 3 adverse events were pyrexia, fatigue and diarrhea. Bearing in mind this was a heavily pre-treated population, many of whom had received prior immunotherapy, I thought the results were promising. The downside of immunotherapy is that while it has shown effectiveness, it does leave patients, especially the elderly, rather beaten up. The lack of grade 4 events in CLL was especially encouraging in this group.
What are the main BTK inhibitors in development?
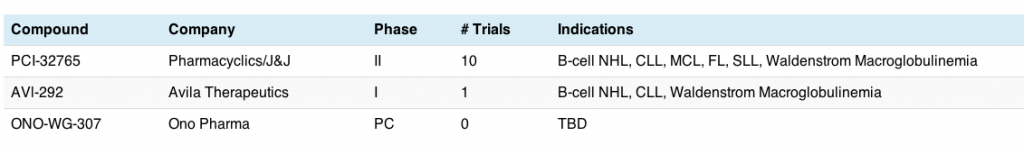
Johnson and Johnson announced they were licensing Pharmacyclics PCI-32765 compound for nearly a $1B just prior to the meeting.6
Based on the data seen over the last two years, I thought they got a steal – this looks like it will be a very promising agent indeed. However, while they are clearly the leading BTK inhibitor, based on the sheer breadth and depth of their program, they aren’t the only one in this niche.
Avila Therapuetics also have a BTKi in early development, AVL-292, but they only have one phase I trial ongoing that I could find. The initial phase I data in B-cell malignancies was also presented in a poster at ASH.7
You can see why J&J licensed the Pharmacyclics agent – it’s much more advanced in the clinic than the others:
What does all this data mean?
Emerging data on BTK inhibitors has started to show that they produce consistently effective and well tolerated agents in B-cell malignancies such as NHL, MCL and CLL. I think this is a new class we are going to hear a lot more about over the next few years, either alone or in combination with other therapies.
2 Responses to “ASH 2011 Update 1: Bruton’s Kinase Inhibitors (BTK)”
[…] Post navigation ← Previous […]
Thanks for the report. It’s as good a (the best actually) description I have found re. this stuff, as I try to weigh the pros and cons of PCI-32765 and AVL-292 (as a patient shopping for a clinical trial).
Comments are closed.