Ribonucleases (RNase) – clinical applications for cancer therapy
This is the second post of a two-part mini series on RNases with Dr Laura Strong of Quintessence Biosciences. If you haven’t yet read it, check out yesterday’s post, which focused on Ribonucleases (RNase) – what are they and why are they relevant to cancer?
Yesterday, we learned that RNases kill cancer cells by a novel mechanism – destruction of RNA – and may be synergistic with some chemotherapy agents.
In the second part of the mini-series, Laura is going to discuss Quintessence’s progress with moving QBI–139, their lead RNase compound, from precinical research to the clinic. This post focuses on how a small biotech company decided upon the relevant clinical targets they wanted to focus on and reported the initial findings at the American Association for Cancer Research (AACR) meeting last month.
What is the clinical plan for a broadly active agent without a marker?
We took QBI–139 into a first-in-human Phase I trial with the primary objective of understanding the safety profile of the drug in patients with solid tumors. While dose escalating continues, the trial should be complete this year.
In the meantime, we have been refining our strategy for the next stage of clinical development. One of our challenges is the selection of tumor type because the drug showed broad efficacy in the xenograft models. After considering a variety of factors (including markets, competition, regulatory impacts and clinical trial designs), we narrowed our disease focus to non-small cell lung (NSCLC) and ovarian cancers. Despite having single agent activity, we anticipate advancing QBI–139 as part of a combination regimen with a standard of care drug. We have been gathering in vitro and in vivo data to support these approaches and we shared some of our in vitro results at the AACR 2012 annual meeting.
To select the drugs we would combine with QBI–139, the first, second and third line therapies in non-small cell lung and ovarian cancers were evaluated. The diseases are an interesting dichotomy because ovarian cancer is still largely treated as a single disease while non-small cell lung cancer (NSCLC) is transitioning to a collection of diseases divided largely by genetic mutations with some differences based on histology.
First line therapies in ovarian cancer are based on combinations of platinum drugs and taxanes. In contrast, second and third line therapies for ovarian cancer involve a variety of drugs (e.g. topotecan, gemcitabine, vinorelbine), which resulted in selection of cisplatin and docetaxel to explore in combination with QBI–139.
NSCLC patients with changes in EGFR, KRAS and ALK will be treated with targeted agents as first line therapy. The remainder, which is actually the majority, of NSCLC patients, will receive a first line therapy that often includes cisplatin as part of a combination regimen. {Editor’s Note: common NSCLC therapies typically include a platinum (eg cisplatin or carboplatin) and a taxane (eg paclitaxel or docetaxel), or other chemotherapy doublets (eg gemcitabine or pemetrexed with a platinum.)}
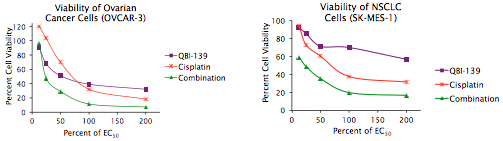
A cell viability assay was run to determine the concentration of each single agent that caused a half maximal effect (EC50). The combination studies were then run starting with each drug at the concentration of maximal effect.
Two graphs are provided as examples of the results. The ovarian cancer cell line OVCAR–3 (left) and the NSCLC cell line SK-MES–1 (right) were treated with QBI–139, cisplatin or a combination of the two drugs. The QBI–139 + cisplatin had an additive effect on the OVCAR–3 (ovarian cancer) cells and a synergistic effect on the SK-MES–1 (NSCLC) cells.
The combination index (CI) is then determined using the median effect analysis (This approach is sometimes referred to as the Chou Talalay combination index.). The CI values represent: additive effect (CI = 1), synergy (CI < 1) and antagonism (CI > 1).
What combinations have been evaluated so far?
The QBI–139 combinations showed synergy or additive effects against the ovarian cancer lines tested:
QBI–139 + Cisplatin:
- SKOV–3 cells: CI=0.33
- OVCAR–3 cells: CI=1
QBI–139 + Docetaxel:
- SKOV–3 cells: CI=0.037
- IGROV–1 cells: CI=0.05
The QBI–139 + cisplatin combination was synergistic against the non-small cell lung cancer lines tested:
- A549 cells: CI=0.714
- SK-MES–1 cells: CI=0.4
So what comes next for RNase therapies?
The discovery that naturally occurring RNases could be exploited for potent anti-cancer drugs has provided an alternative approach to RNA as a therapeutic target. Our efforts have advanced a drug with broad activity in xenograft models into the clinic. As we complete the Phase I trial, we are working to best position the drug for the next step on the path to delivering a new tool to help cancer patients.
One Response to “Ribonucleases (RNase) – clinical applications for cancer therapy”
Really interesting and informative post. I will read the other posts of you as well. Thank you for sharing such a valuable information with us.
Comments are closed.