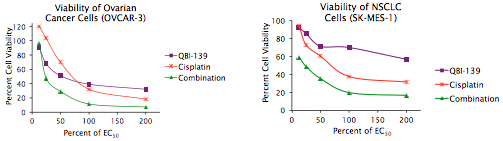
ASH 2012 update on ARRY-520 in multiple myeloma
After highlighting the interesting biomarker program associated with AVEO’s tivozanib in renal cell and triple negative breast cancers in the last post, several people wrote in asking about other biomarker programs that have piqued my interest. Regular PSB readers will know that I’m not a fan of catch-all trials at all because the population being studied is too heterogeneous – use of biomarkers can help select which patients are more likely to respond to a particular drug and thus produce greater efficacy.
Another small biotech doing some interesting and compelling biomarker work is Array BioPharma, based in Boulder, Colorado.
What’s exciting about the ARRAY pipeline?
They have a nice pipeline of interesting targeted agents that are being developed with several big Pharma partners including AstraZeneca (selumetinib) and Novartis MEK162), both MEK inhibitors. Dennis Slamon’s lab has published some initial data on their work on predictive biomarkers with selumetinib for those interested (see References below).
MEK inhibitors are thought to target KRAS, which causes resistance in lung cancer and melanoma. Combining MEK with a PI3K inhibitor may reduce the adaptive resistance and prolong survival. I’m expecting to see more on these at the forthcoming AACR and ASCO meetings in April and May. The approach is most likely to be incremental rather than a home run, though.
The ARRAY biomarker program that has intrigued me was presented in multiple myeloma (MM) at ASH in December. They have a novel kinase that targets Kinesin Spindle Protein (KSP) codenamed ARRY-520, which has a novel mechanism of action. For those of you interested in learning more about the basic biology of the KSP concept, I highly recommend checking out the papers in the Reference section below as they explain the targeting of the microtubilin concept well and how KSP inhibition differs from what we know of the taxanes as well as some useful background on the compound itself.
Some of you will remember previous KSPs that failed in the clinic such as ispinesib, for example, but not all compounds are equal or destined to fail. Much of cancer research is iterative – tweaking molecules to improve the conformation (shape), reducing side effects or improving potency. Sometimes a whole program can be canned because the company selected the wrong tumour type or trial design – you don’t always get multiple shots on goal in small biotechs!
What’s interesting to me is the focus of Array in multiple myeloma, a particularly difficult and foxy disease to impact clinically. The basic rationale behind this approach is that KSP is a microtubule protein required for mitosis (cell division); inhibition leads to cell death (apoptosis).
Now, remember the bedrock of therapy in MM has been proteasome inhibitors (bortezomib and now carfilzomib have been approved) and Immune Mediated inflammatory Disease agents or IMiDs (thalidomide, lenalidomide and now pomalidomide are all approved). This means that new agents with a different mechanism of action attract a lot of attention, especially if they can potentially be combined with existing drug classes to prolong survival and push patients into minimal residual disease (MRD) where the myeloma clone is drastically suppressed, much as we see in CML with the BCR-ABL inhibitors.
Inevitably, new drugs in MM are tested in a highly refractive population, either as a single agent or in combination with dexamethasone (dex) to determine if they have any efficacy.
Recall that two new agents approved by the FDA in relapsed/refractory MM had relative low single digit response rates (RR) as single agents i.e. carfilzomib (22.9%) and pomalidomide (7.4%) with improved RR in combination with low dose dex i.e. carfilzomib (34%) and pomalidomide (29.2%)
The two ARRY-520 analyses at ASH looked promising

Dr Satin Shah, ASH 2012
The first part of the phase II study from Shah et al., (Abstract #449) looked at patients who were highly refractive to bortezomib and/or lenalidomide.
They observed that ARRY-520 had encouraging efficacy with and without dex. The first cohort (n=32) evaluated single agent therapy with ARRY-520 and saw 19% confirmed responses (CR) and 15% partial responses (PR); the subset who were both bortezomib and lenalidomide refractory had an ORR of 15%.
Meanwhile, the second cohort (n=18) looked at the combination with low-dose dex and demonstrated a PR or better of 22% in a heavily pre-treated population (more than 10 prior regimens). Adverse events included the usual myelosuppression (neutropenia, thrombocytopenia, anemia) seen in myeloma, but importantly, no treatment related neuropathy was observed.
The second element of the analysis looked at the same patients, but described the results from a biomarker, alpha-1-acidic glycoprotein (AAG). AAG is a serum protein that can have elevated levels in MM. As Dr Shah noted, AAG is not known to bind to standard of care agents in MM but is thought to bind to ARRY-520 with a negative impact by reducing the available amount of drug. In other words, they have a sub-therapeutic exposure to ARRY-520.
In the phase II study, they found that the hypothesis was supported: patients with high levels of AAG had poorer responses, while patients without elevated AAG levels had much better responses and the ORR increased to 33%, a dramatic improvement. The pre-dose AAG levels therefore correlated with response.
Obviously, the biomarker will need to be validated in larger, randomized controlled trials, but it would be very useful to be able to select patients upfront who could receive ARRY-520 either in combination with low dose dex or with a proteasome inhibitor or IMiD and see a more pronounced response. An initial trial with carfilzomib suggested an acceptable toxicity profile, while this is an encouraging start, we still need more data on the safety and efficacy of the combinations going forward.
Assuming the ongoing Phase Ib combination trials demonstrate good tolerability and efficacy, ARRY-520 could be potentially be combined with dexamethasone and either carfilzomib or pomalidomide in the relapsed/refractory setting for greater responses than the doublets alone.
Some additional thoughts…
I thought the Array KSP compound looked very encouraging indeed – multiple myeloma is crying out for:
- New agents with a different mechanism of action from the existing standards of care that can be combined to give solid results from triple combinations.
- More competition in the refractory setting to push out the MOS further and try to achieve minimal residue disease (MRD), which would impact the lives of patients with multiple myeloma significantly.
Beyond Kyprolis and Pomalyst, here are other agents in phase III studies being tested in refractory MM such as the HDACs e.g. panobinostat and vorinostat. The vorinostat data presented at ASH in 2011 was singularly disappointing, but hopefully we will hear about the phase III panobinostat results later this year.
In the meantime, Array have a nice compound in ARRY-520 and a potentially useful biomarker of response to help select patients upfront who are more likely to respond to treatment. As far as I know, they don’t yet have a partner for the program and may well need one for the large phase III trials that will be needed for FDA approval or they may well try to go it alone using the capital raised from the MEK partnerships. MM is certainly a promising avenue worth exploring for ARRY-520 and I look forward to hearing more about its development.
References:
![]() Garon, E., Finn, R., Hosmer, W., Dering, J., Ginther, C., Adhami, S., Kamranpour, N., Pitts, S., Desai, A., Elashoff, D., French, T., Smith, P., & Slamon, D. (2010). Identification of Common Predictive Markers of In vitro Response to the Mek Inhibitor Selumetinib (AZD6244; ARRY-142886) in Human Breast Cancer and Non-Small Cell Lung Cancer Cell Lines Molecular Cancer Therapeutics, 9 (7), 1985-1994 DOI: 10.1158/1535-7163.MCT-10-0037
Garon, E., Finn, R., Hosmer, W., Dering, J., Ginther, C., Adhami, S., Kamranpour, N., Pitts, S., Desai, A., Elashoff, D., French, T., Smith, P., & Slamon, D. (2010). Identification of Common Predictive Markers of In vitro Response to the Mek Inhibitor Selumetinib (AZD6244; ARRY-142886) in Human Breast Cancer and Non-Small Cell Lung Cancer Cell Lines Molecular Cancer Therapeutics, 9 (7), 1985-1994 DOI: 10.1158/1535-7163.MCT-10-0037
Sarli, V., & Giannis, A. (2008). Targeting the Kinesin Spindle Protein: Basic Principles and Clinical Implications Clinical Cancer Research, 14 (23), 7583-7587 DOI: 10.1158/1078-0432.CCR-08-0120
Tunquist, B., Woessner, R., & Walker, D. (2010). Mcl-1 Stability Determines Mitotic Cell Fate of Human Multiple Myeloma Tumor Cells Treated with the Kinesin Spindle Protein Inhibitor ARRY-520 Molecular Cancer Therapeutics, 9 (7), 2046-2056 DOI: 10.1158/1535-7163.MCT-10-0033


 Following on from my preview of the
Following on from my preview of the 
 At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.
At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.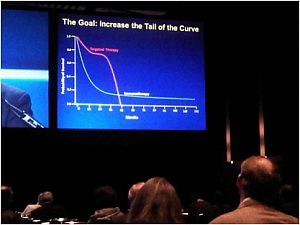 The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.
The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.