One of the nicest things about about going to scientific meetings is that you get to meet interesting people. Instead of swapping cards and going about your way, in the modern world social media allows you to stay in touch more frequently personally while sharing cancer and scientific information over time. The discussions can be very insightful and enriching.
Two recent PhDs from MD Anderson Cancer Center (MDACC), Drs Angela Alexander and Jeannine Garnett are two of those research scientists I met while at cancer meetings and now keep in touch with – congratulations on your well earned doctorates this summer, ladies!
Angela recently started a nice blog The Cancer Geek and posted an extensive review of how she uses her iPad during the day, including a summary of some of her favourite apps. Check it out, it’s well worth reading!
Funnily enough, I use many of the same apps and a few different ones too. Not surprisingly, our workflow is a little different, given the diversity of things we’re both involved with. Since there’s no active bench research here at Icarus, there’s no need to order supplies. However, I thought for fun it would be a idea to take a leaf out of Angela’s book and take look at what’s on my iPad and how I use it. Here’s my home screen:

After much trial and error, I decided to keep the top most used apps on the main screen (they do change over time with usage and circumstances eg the ESPN Fantasy Football app disappears when the season is over) and organise all the other less used ones into folders in a library screen. On the other two screens I dragged and dropped things into folders such as tools, productivity, books, audio etc and use Search on the home screen to find things quickly like this:
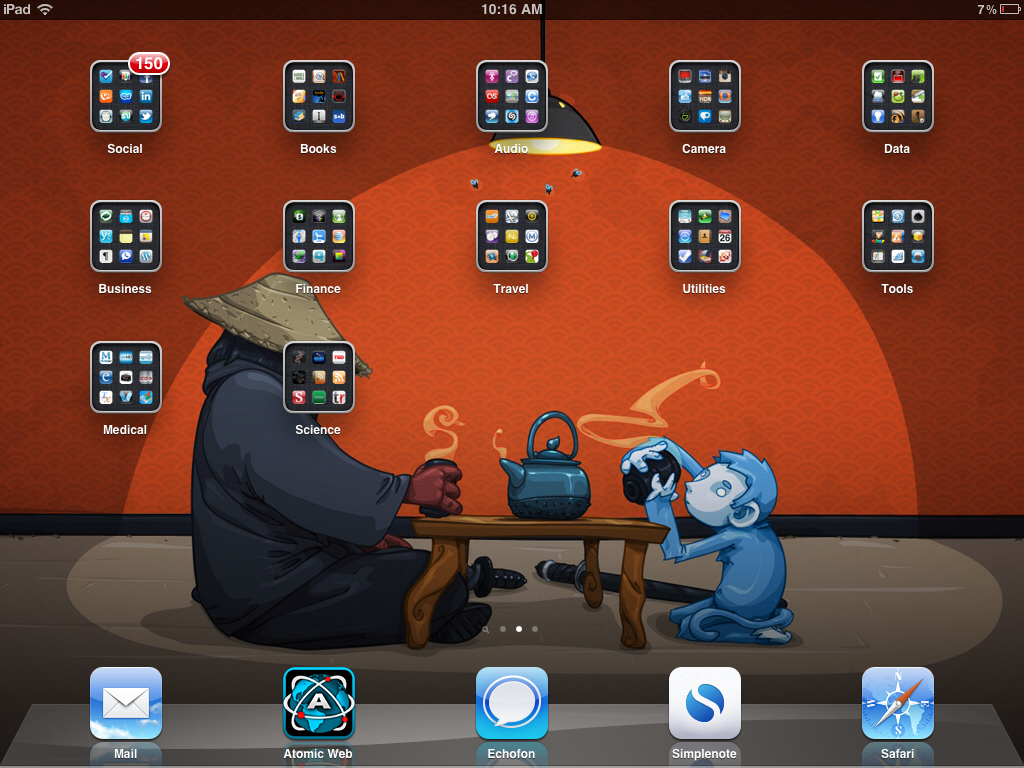
This is much more productive than scrolling through looking for the app you need in a multitude of folders. The app usually comes up in the list after 2-3 letters are typed. Some basic caveats – at home or in the office the iPad is mainly used as a consumption devise to browse (on Safari or Atomic), read (iBooks, Goodreader, AACR Journals), check RSS and blog feeds (BW RSS, Feedly, Reeder), see what hot in Twitter (Echofon and Flipboard) or listen (iPod or Spotify for music, Science podcasts, AACR Webcasts app, Instacast for podcasts such as one of my favourites, MacPowerUsers).
The reason for focus on consumption over creation is that the desktop and laptop are much more powerful and heavy duty for content creation. However, there are some cool tools for capturing drawings and scribbles such as Penultimate and Listary, which is excellent for syncing a quick To-Do list between the iPad and iPhone.
On the road though, the iPad becomes a very nifty and efficient creation tool that fits my workflow at scientific congresses nicely. Yes, I have taken just the iPad to conferences and left the laptop behind without much difficulty. That was liberating!
There are some nifty productivity To Do apps out there such as Things and OmniFocus (I prefer the latter) in addition to password security, which I highly recommend in case your iPad goes missing – 1Password is my personal favourite.
One important point to note – I truly detest the shackles of Microsoft Office and have never been a big fan of the bloatware it has become. Years ago, while doing my PhD, the Physiology unit started migrating from WordPerfect, with its fast keyboard shortcuts and better graphic integration tools, to Word. The constant fiddling with autochanges to formatting, size and scaling drove me potty then and I still scowl if I have to use any of the apps with the exception of Excel, although the simplicity of Lotus 1-2-3 and its macros and keyboard slashkeys is a happy memory to this day.
PC and Windows users are very much stuck in the world of files organised into folders, but many Mac tools and the iPad in particular don’t work this way, so the thinking behind it is rather different. Think cloud apps and sync through Dropbox or searching for things based on Tagging, much in the same way that other apps such as Gmail, various text editors and Evernote work. This is the way of the future for many and is a much more efficient way to find and store data.
With this in mind, most of my writing (and I do a lot of it as a consultant and blogger) is done in plain text – simple, elegant and infinitely more useful. It took me a while to settle down with one system, but now I’m very happy with Simplenote on the iPad, iPhone or desktop and Notational Velocity Alt (nvALT) on the desktop. They sync beautifully together once you enter your username/password. This guarantees a natural backup will always be there. Some of the data is also synced to Dropbox.
Surprisingly, I now have thousands of blog posts, snippets, text and notes accrued in this handy text sync system. While walking around at cancer meetings, I take quick notes of interesting things from the posters or add quotes from chats with presenters straight into Simplenote on the iPad. For oral presentations, these go much faster than my typing skills allow, so I write long hand in my Moleskine and add notes manually in a quiet moment later so that they become searchable and re-usable.
John Gruber’s awesome Markdown syntax (discussed in Daring Fireball) and Fletcher Penny’s Multimarkdown are tools I make use of a lot, especially as conversion tools allows me to preview the text and then convert it to html for cutting and pasting into WordPress, the platform used for this blog. Text Expander Touch on the iPad uses the same master shortcut file as the desktop/laptop versions and makes repetitive typing of various tumour types, for example, so much faster!
Text or RTF files created in apps on the iPad can be synced via Dropbox for later use and aggregation in various desktop apps. I save Markdown notes and snippets as text files in Simplenote or Elements and once on a laptop, drag them to Marked, a cocoa desktop app from Brett Terpestra to preview and convert into html for blog posts or text for reports.
Scrivener is my Word Processor of choice these days, not Word, because it is simply superb for technical research and writing. I can’t wait for the makers to come out with an iPad app! For now, I use different creating tools on the iPad since Scrivener supports a host of different inputs from TXT or RTF files that have been created on the iPad, whether from Simplenote, Index Cards (great for creating an outline), iThoughtsHD (a mind map tool) or Evernote, where I clip and store interesting websites.
Creating short and long form articles, posts and reports is really easy and much faster in Scrivener when you are focusing on the content and not the formatting. Since the export function is very versatile, you can also create different documents formatted as PDFs, LaTeX or epub format (for the Kindle), whichever you choose to apply. Overall, I have found this tool to be extremely versatile and saves me a lot of time.
Other iPad apps I enjoy using include iKinase, which provides a handy tool for finding protein and chemical structures for small molecules and Molecules, which shows 3D molecular structures that you can manipulate with your fingers – very cool.
When travelling, I love the TripIt app for keeping me straight on flight and hotel details, which it picks up from emails sent to my business account and creates itineraries automatically. On the road, though, I tend to use the iPhone more than the iPad for viewing the details as it is smaller and more mobile while waiting at a taxi line. The maps produced from the destination are useful for finding your way around or telling the cab driver where to go!
Another app I have on both my iPhone and iPad are QR code readers but the reality is that it’s much easier to get the code from an iPhone, especially if the code is awkwardly placed on a poster.
Need an app for curating your expenses on the road? iXpenseIt is useful for tracking cash receipts rather than losing or forgetting about them. Many banks also now have iPhone and iPad apps that are worth checking out for scanning checks and checking expenses.
Wondering what one of my favourite apps is? Try the Howard Hughes Medical Institute (HHMI) quarterly bulletin – a truly beautiful combination of art and science that is a pleasure to read. Reading medical journals on the iPad is also a delight – I read Nature, NEJM and AACR journals regularly on the iPad and download articles as PDFs for easy reading offline while travelling in iBooks.
Overall, I love my iPad as a consumption tool and travel with it regularly to cancer conferences along with my Moleskine and also a laptop for more heavy duty work. There is something about the iPad that makes it hard to put down.