Metastatic melanoma – what’s new on the horizon?
Metastatic melanoma is quite a hot topic right now with a rich pipeline of products in development after a decade of little or no progress. Of course, it is a bit like three London buses coming along at once after an hour long wait in the winter weather, but better late than never.
Many of you will remember the recent data from ipilimumab (BMS), an immunotherapy that showed increased survival, albeit with some severe adverse events, from the phase III trial in newly diagnosed metastatic melanoma presented at ASCO in the plenary session earlier this year, followed by a publication in the NEJM. The FDA filing was subsequently submitted on the basis of the positive data.
Yesterday, BMS announced that the FDA have moved the PDUFA date back 3 months from Dec 25th to March 26th, 2011. A precise reason for the delay wasn’t given , but the company did say:
“In response to an FDA request, Bristol-Myers Squibb submitted further analysis of data pertaining to the current application for pre-treated advanced melanoma and the agency considers this to be a major amendment to the drug’s BLA.”
I’m not going to speculate on the reasons for the extra review time or what the new data was, but it is an interesting and unexpected development.
Meanwhile, there’s also been a lot of buzz around targeted BRAF inhibition in melanoma lately, specifically around the initial stunning results seen with PLX4032 (Plexxikon & Roche). So far, it seems that responses of around 6-12 months, with a median of around 8 months are possible with an kinase inhibitor that specifically targets the V600E mutation associated with BRAF, although there two problems:
- The responses are not durable as resistance (eg associated with MEK or AKT amplification) sets in.
- Inhibiting CRAF as well as BRAF appears to lead to an unwanted excess proliferation of squamous cells, which is reversible on withdrawal of treatment.
In the first case, a couple of recent papers have looked at mechanisms of resistance around BRAF inhibition that give us some clues of where to go next.
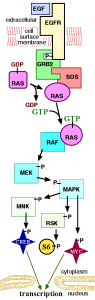 Gopal et al., (2010) decided to see what happened with AZD6244 or selumetinib (Array and AstraZeneca), a MEK and MAP/ERK inhibitor, and whether it would have any impact in mitigating BRAF resistance, given the potential close interaction within the RAS-RAF-MAPK pathway and downstream events that could be impacted through cross-talk and feedback loops:
Gopal et al., (2010) decided to see what happened with AZD6244 or selumetinib (Array and AstraZeneca), a MEK and MAP/ERK inhibitor, and whether it would have any impact in mitigating BRAF resistance, given the potential close interaction within the RAS-RAF-MAPK pathway and downstream events that could be impacted through cross-talk and feedback loops:
“We analyzed a panel of Braf mutant human cutaneous melanoma cell lines for their sensitivity to growth and survival inhibition by AZD6244. We compared these effects with the baseline activation status of signaling pathways in the cells, and with AZD6244 treatment–induced changes in signaling networks.
These studies have identified the phosphoinositide 3-kinase (PI3K)-AKT pathway as a critical regulator of the efficacy of AZD6244 in Braf-mutant melanomas, including in cells without baseline activation of the pathway.”
In order to determine possible mechanisms of resistance in the cell lines, they compared the effects of AZD6244 treatment on their signaling pathways with effects in sensitive cell lines and found:
“Although all four of these Braf-mutant cell lines showed similar degree and duration of MAPK inhibition and several other proteins, the resistant cell lines increased their P-AKT levels following exposure to AZD6244, which was not observed in the sensitive cell lines.”
They went on to note:
“The functional significance of AKT activation is supported by the fact that inhibition of AKT activity, either by AKT knockdown or concurrent treatment with the mTORC1/2 inhibitor AZD8055, resulted in synergistic cell killing in the resistant cell lines.”
AstraZeneca and Merck have an ongoing partnership with their MEK (AZD6244) and AKT (MK-2206) kinase inhibitors, so combining them in a clinical trial to try and reduce resistance via feedback loops here would be an interesting approach worth trying. Such a combination trial is currently recruiting in advanced solid tumours, not melanoma per se. It is, however, a classic catch-all phase I study to see what kinds of cancers might respond and determine the MTD, but I would be very interested to see the data from patients with metastatic melanoma if they are enrolled.
Now, it has been shown in breast cancer cell lines showed that MEK inhibition resulted in cross-activation of the EGFR tyrosine growth factor receptor, but EGFR has not been shown to be relevant in melanoma, so Gopal et al., considered what other receptors might be responsible for mediating the effects. In the discussion, an interesting snippet caught my eye:
“AZD6244 treatment induced a slight increase of IGF-I secretion by the cells, and knockdown of IGF-I also blocked P-AKT induction by AZD6244. Supporting a specific role for the pathway in cell survival, recombinant IGF-I treatment blocked AZD6244-induced cell death, but not growth arrest, in the sensitive WM35.”
This might also suggest another useful combination approach to consider in clinical trials.
Previously, it has been shown that targeting BRAF can not only inhibit the important driver in melanoma, the V600E mutation, but it can also stimulate cellular signaling through the MEK-ERK pathway by activating the related family member C-RAF. This may explain the squamous cell proliferation seen in some patients with PLX4032. The more ideal BRAF inhibitor would therefore specifically target BRAF V600E, without activating CRAF at the same time.
Related to the subject of malignant melanoma, Kamata et al., (2010) just published a paper that looked at the relationship between BRAF and CRAF in the disease. Previously it has been shown that D594A BRAF lacks kinase activity, but can induce the related gene product CRAF in addition to the mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK pathway. What they found was really interesting. In a nutshell:
“We show that the aneuploid phenotype is dependent on Craf. Treatment with the MEK inhibitor U0126 did not attenuate the emergence of aneuploidy but prevented the growth of aneuploid cells. These results provide a previously unidentified link between Craf and chromosomal stability, with important implications for our understanding of the development of cancers with driver mutations that hyperactivate Craf.”
Aneuploidy is an abnormal number of chromosomes and can lead to genetic instability, a key cancer hallmark. It’s an important concept here because Kamata et al., have offered a different reason for the CRAF proliferation observed with some BRAF inhibitors:
“Impaired activity BRAF mutants are frequently coincident with oncogenic RAS mutations in human cancers (26) and in these, albeit rare, cancers, we may expect the hyper-activated CRAF induced by the combination of both oncogenes to enhance the aneuploidy response compared with mutation of either oncogene alone. Such a situation is likely to be highly detrimental to the individual and, indeed, this mechanism may well account for the highly aggressive melanomas we observed following the combined expression of D594A Braf and G12D Kras in melanocytes.”
All in all, this is a very complex yet fascinating area of research and for those of you interested in this field, I would highly recommend reading the latest papers.
Photo Credit: Wikipedia
References:
![]() Boni, A., Cogdill, A., Dang, P., Udayakumar, D., Njauw, C., Sloss, C., Ferrone, C., Flaherty, K., Lawrence, D., Fisher, D., Tsao, H., & Wargo, J. (2010). Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function Cancer Research, 70 (13), 5213-5219 DOI: 10.1158/0008-5472.CAN-10-0118
Boni, A., Cogdill, A., Dang, P., Udayakumar, D., Njauw, C., Sloss, C., Ferrone, C., Flaherty, K., Lawrence, D., Fisher, D., Tsao, H., & Wargo, J. (2010). Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function Cancer Research, 70 (13), 5213-5219 DOI: 10.1158/0008-5472.CAN-10-0118
Garnett MJ, Rana S, Paterson H, Barford D, & Marais R (2005). Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Molecular cell, 20 (6), 963-9 PMID: 16364920
Gopal, Y., Deng, W., Woodman, S., Komurov, K., Ram, P., Smith, P., & Davies, M. (2010). Basal and Treatment-Induced Activation of AKT Mediates Resistance to Cell Death by AZD6244 (ARRY-142886) in Braf-Mutant Human Cutaneous Melanoma Cells Cancer Research, 70 (21), 8736-8747 DOI: 10.1158/0008-5472.CAN-10-0902
Kamata, T., Hussain, J., Giblett, S., Hayward, R., Marais, R., & Pritchard, C. (2010). BRAF Inactivation Drives Aneuploidy by Deregulating CRAF Cancer Research, 70 (21), 8475-8486 DOI: 10.1158/0008-5472.CAN-10-0603
4 Responses to “Metastatic melanoma – what’s new on the horizon?”
[…] This post was mentioned on Twitter by Sally Church, Patrick McCann. Patrick McCann said: RT @MaverickNY: An update on the exciting developments in melanoma and new options for the future: http://bit.ly/aLIYoI $RHHBY $BMY $AZN … […]
Sally, you left out Provectus Pharmaceuticals and their trials with PV-10 and melanoma. They are presenting all data from PII trial this week in Sydney. Keep an eye out for it. You may find it interesting.
Hi Eileen,
Yes I did leave PV-10, mainly because I haven’t seen much new data since ASCO 2009 (cf http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=34524) but like you, I’ll be watching to see what the phase II data looks like.
It’s also chemoablation rather than targeted therapy, so much less interesting to me scientifically.
[…] # As background, you can read up on the past developments with BRAF V600 mutated melanoma here, here and here, including the phase II NEJM data, mechanisms of resistance (MEK or AKT) and how […]
Comments are closed.