Update on ADCs and immunotoxins in hematologic malignancies
The Cancer Research journal has an interesting review of ADC and immunotoxin technology and their potential role in hematologic malignancies that is well worth reading (see references below).
Fitzgerald et al., (2011) observe that:
Immunotoxins and ADCs are assembled in a number of different ways. Antibody fragments or whole antibodies are combined with either protein toxins or low-molecular-weight toxic drugs. Linkage options include gene fusions (peptide bonds), disulfide bonds, and thioether bonds.
Design goals dictate that immunotoxins and ADCs remain intact while in systemic circulation but disassemble inside the target cell, releasing the toxic payload.
Uncoupling the toxin or drug from the antibody is accomplished by protease degradation, disulfide bond reduction, or hydrolysis of an acid-labile bond. Toxin or drug attachment to the antibody must not interfere with antigen binding.
I was particularly struck by the number of immunotoxins and ADCs currently in clinical development each focused on different targets. The article highlights a number of agents in the clinic, as of January this year.
A quick overview is provided in the table below:
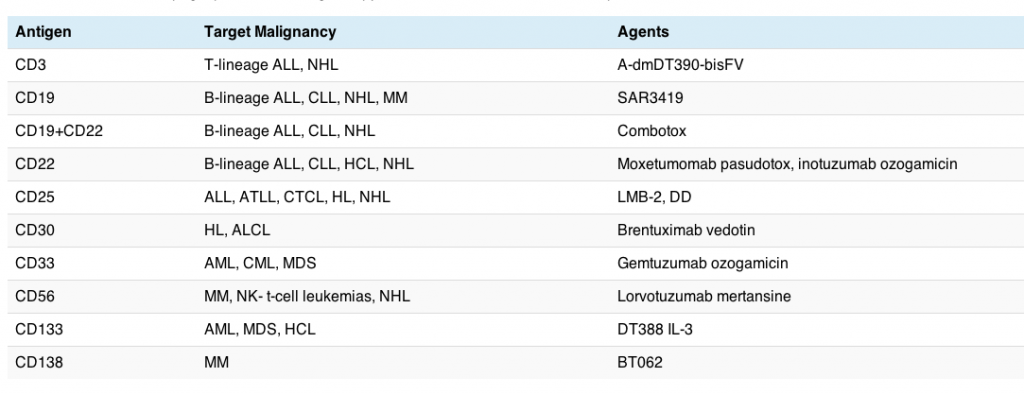
The good news is that some of these agents have already been approved in the US, although not all have seen a successful launch. While the current rising star from Seattle Genetics and Millennium, brentuximab vedotin (Adcetris), received fast track approval from the FDA in HL and ALCL earlier this year, gemtuzumab ozogamicin (Mylotarg) was voluntarily withdrawn from the US market by Pfizer last summer. Part of Mylotarg’s problems lay in the early generation linker technology that release the active substance too early, thereby causing more extensive side effects than would be preferred. Linker technology has evolved considerably since then, which is good news for patients since active drug can now be targeted more precisely where it is most needed.
As the antibody and linkage technology continues to improve, thereby allowing the active drug to be released in the tumour or on the surface of the cancer cells, I would expect to see more of these agents make it to market successfully with fewer toxicities.
One promising agent in this list, moxetumomab pasudotox (MedImmune), had some promising early data presented at ASH in 2010 in relapsed, refractory hairy cell leukemia (HCL) that is worth checking out.
The regular deadline for this years ASH abstracts has already closed, while the late breaker deadline is this month. I’m hoping we will see some more promising data emerge in December.
References:
![]() FitzGerald, D., Wayne, A., Kreitman, R., & Pastan, I. (2011). Treatment of Hematologic Malignancies with Immunotoxins and Antibody-Drug Conjugates Cancer Research, 71 (20), 6300-6309 DOI: 10.1158/0008-5472.CAN-11-1374
FitzGerald, D., Wayne, A., Kreitman, R., & Pastan, I. (2011). Treatment of Hematologic Malignancies with Immunotoxins and Antibody-Drug Conjugates Cancer Research, 71 (20), 6300-6309 DOI: 10.1158/0008-5472.CAN-11-1374