Update on emerging therapies in ALK+ lung cancer
Last week’s post on FGFR1 mutations in squamous cell lung cancer and the new EGFR/ALK combination agent, AP26113 (Ariad) drew a lot of attention from readers, with many writing in for more details or correctly suggesting an update in squamous cell carcinoma was long overdue.
Oddly, there wasn’t much in the way of new or exciting data in lung cancer at the recent American Society of Clinical Oncology (ASCO), but there are some recent developments that are worth looking at in EGFR, ALK+ and squamous cell carcinoma.
Let’s take a look at each in turn over a series of blog posts.
In the old days, lung cancer was first divided into Small Cell Carcinoma (SCC) and Non-Small Cell Lung Carcinoma (NSCLC), which have a broad split of about 20:80. As we learned more about the disease, NSCLC was eventually divided further in squamous and non-squamous histology, as therapies such as pemetrexed (non-squamous) and bevacizumab (non-squamous) emerged.
Erlotinib was found to work best in adenocarcinomas, ie EGFR mutation-positive tumours. As far as I know, there are no approved targeted therapies for the treatment of squamous histology and much of the focus has been in mutations associated with adenocarcinomas, which mostly (but not always) tend to be associated with non-smokers.
ELM4-ALK translocations
Within adenocarcinomas, we are learning that EGFR isn’t the only mutation that might be a potential target, as the recent data in crizotinib published in the NEJM by Kwak et al., (2010) on ELM4-ALK translocations has shown.
For those of you interested in the development of the ALK translocations in lung cancer, Dr Ross Camidge also provided an excellent overview when we interviewed him on Pharma Stratgy Blog last year. It’s worth checking out if you missed it, and has become one of our most popular posts since last October.
Dr Jack West from GRACE has diligently curated a huge volume of posts, interviews and webcasts on lung cancer, including this nifty chart showing the currently identified mutations in adenocarcinomas:

Source: GRACE
Of course, as luck would have it, EGFR mutations and ALK translocations tend to be mutually exclusive, so there would probably be little benefit in combining agents that target EGFR or ALK mutations, even in adenocarcinomas.
Crizotinib, the first ALK inhibitor to successfully make it past phase II trials, has already been filed by Pfizer for approval with the FDA and should provide a new option for lung cancer patients with this translocation very soon. This is an exciting development because oncologists will be able to order a FISH test using the companion diagnostic developed by Abbott (also submitted to the FDA) to determine if their patients will be suitable for crizotinib therapy.
Although crizotinib was originally developed as a c-MET inhibitor, its activity there was very weak (the Roche and ArQule compounds, MET-Mab and ARQ197 respectively, are much more potent and continue to look promising in phase II trials), the discovery of the ALK translocation changed the clinical development plan dramatically and for the better.
Unsurprisingly, there aren’t too many ALK inhibitors in development to date, with crizotinib being the lead compound:
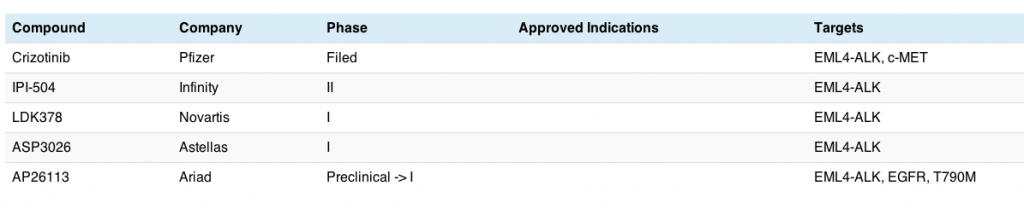
This is a small, but rapidly growing niche; already we can see that compounds are emerging into the clinic hot on crizotinib’s heels. The Infinity compound is a little different – it’s a heat shock protein (Hsp), while both the Novartis and Astellas agents are small molecule TKIs. As far as I know, there isn’t a monoclonal antibody or antibody drug conjugate in the clinic for this particular target yet.
Like crizotinib, AP26113 is also a small molecule TKI, but differs in that it appears to be a dual inhibitor of ALK and EGFR, including the T790M mutation that has been shown to confer resistance to EGFR inhibitors such as erlotinib in adenocarcinomas (see Hammerman et al., 2009).
Conclusions
The time between the discovery of the ELM4-ALK translocation in adenocarcinomas and moving crizotinib into clinical trials was pretty rapid, and a tribute to Pfizer’s scientists and clinicians who made that happen so expeditiously. It will be interesting to how this niche develops once FDA approval has occurred, and whether the other inhibitors in development will be merely ‘me-too’ agents or able to raise the bar beyond crizotinib in terms of efficacy, safety or overcoming resistance due to the structure forming a different binding shape in the kinase domain. Time will tell.
Disclosure: I am an unpaid member/volunteer of the GRACE Board.
{UPDATE: Thanks to Luke Timmerman of Xconomy tweeting about Tesaro, I noticed they now have a deal as of March with Amgen for unnamed ALK inhibitors in their pipeline.}
References:
![]() Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, & Iafrate AJ (2010). Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England Journal of Medicine, 363 (18), 1693-703 PMID: 20979469
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, & Iafrate AJ (2010). Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England Journal of Medicine, 363 (18), 1693-703 PMID: 20979469