Is NOTCH a new target in leukemia?
Over the last five years I’ve been involved in quite a bit of market research relating to chronic lymphocytic leukemia (CLL) and have been struck how the core therapies are still largely chemotherapy based. CLL is an indolent disease of the bone marrow, where it produces too many lymphocytes (white blood cells), whereas chronic myeloid leukemia (CML) is an excess production of myeloid cells:

Source: cancer.gov
Interestingly, while we know a fair bit about the biology of CML in terms of the driver mutation, ie Philadelphia chromosome that occurs as a result of the translocation from the 9 and 22 chromosomes thereby producing a new gene, BCR-ABL, we don’t half as much about the underlying biology pertaining to CLL.
Compare the simple elegance of CML biology to CLL, as Fabbri et al., (2011) noted:
“The pathogenesis of chronic lymphocytic leukemia (CLL), the most common leukemia in adults, is still largely unknown. The full spectrum of genetic lesions that are present in the CLL genome, and therefore the number and identity of dysregulated cellular pathways, have not been identified.”
This means that in CML, we have a valid target (BCR-ABL) and several highly effective tyrosine kinase inhibitors (TKIs) are now approved to treat the disease, turning CML from a certain death sentence into a cancer now be managed as a chronic condition.
By contrast, in CLL (which tends to have an older population than CML), we largely stuck with various immunotherapies and chemotherapies such as:
- fludarabine (F)
- cyclophosphamide (C)
- bendamustine/Treanda (B)
- chlorambucil/Leukeran (L)
- doxorubicin/Adriamycin (A)
These are often used in combination with each other, or with the monoclonal antibody, rituximab (R) eg FCR, FC, FR, BR etc.
Other monoclonal antibodies in use include alemtuzumab (Campath) and ofatumumab (Arzerra), although these are often reserved for the refractory setting because FCR or FC or FR are usually preferred upfront, with BR often preferred as second line therapy. Alternative therapies that are beginning to emerge in clinical practice in the refractory setting (based on clinical trials) are bortezomib (Velcade) and lenalidomide (Revlimid), although both are currently approved for multiple myeloma and not CLL.
The challenge though, is the same old chestnut that exists for many tumour types – a heterogeneous disease without clear molecular targets means that patients cycle through various chemotherapies or immunotherapies, which prolong outcomes at the cost of relatively poor quality of life due to the extensive side effects of chronic treatment that ends up with weary, beaten up and worn out people.
With that somewhat depressing landscape in mind, my interest was piqued by Fabbrio et al’s (2011) paper on CLL and mutations that has just been published this week.
What’s new in CLL?
Basically, the group undertook an analysis to look at the mutations found in the genes of CLL patients at different stages of the disease. They found several mutations not previously linked with CLL, but most patients had relatively few genetic mutations compared to some other types of cancer.
However, they did find something very interesting:
“Mutational activation of NOTCH1, observed in 8.3% of CLL at diagnosis, was detected at significantly higher frequency during disease progression toward Richter transformation (31.0%), as well as in chemorefractory CLL (20.8%).”
That was one of those “ooh” moments that made me read on and see what else they have to say and what the implications are. Is NOTCH1 a good molecular target that might change things for people with CLL for better?
They went to say:
“Consistent with the association of NOTCH1 mutations with clinically aggressive forms of the disease, NOTCH1 activation at CLL diagnosis emerged as an independent predictor of poor survival.”
A dysregulated pathway can be a useful target to start with, but there are no guarantees, since it may turn out to be a passenger rather than a driver mutation. The only way to find out is to see what happens in clinical trials with CLL patients and to determine what combinations might be useful.
Clinical trials with NOTCH inhibitors
There are currently 54 trials ongoing with NOTCH inhibitors, either alone or in various combinations. Roche have the lead compound in this area, with a broad and deep program already established across multiple potential indications. The table below shows the key players, their NOTCH/gamma secretase inhibitors and the tumour types being evaluated:
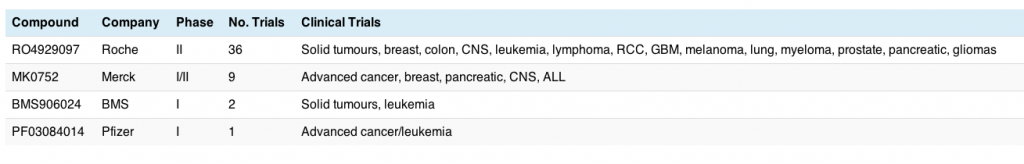
This is still in very early clinical research with mostly phase I (solid tumours or advanced cancers) and only a few phase II trials started, so the race is wide open. Breast cancer and leukemia appear for two of the compounds, although I should point out that the latter tend to be in Acute Lymphoblastic Leukemia (ALL) rather than CLL. This may change with time.
This should be an interesting field to follow and I look forward to writing more about the class as the data matures…
{Update: The Howard Hughes Medical Institute send me a link via Twitter, showing that NOTCH may also have a role to play in a rarer form of leukemia, Chronic Myelomonocytic Leukemia (CMML). They also have a very cool (and free) app for ipods and ipads, which allows you to read their quarterly magazine online. It’s beautifully produced and I very much much enjoy reading it – if only it came out monthly :)}
References:
![]() Fabbri, G., Rasi, S., Rossi, D., Trifonov, V., Khiabanian, H., Ma, J., Grunn, A., Fangazio, M., Capello, D., Monti, S., Cresta, S., Gargiulo, E., Forconi, F., Guarini, A., Arcaini, L., Paulli, M., Laurenti, L., Larocca, L., Marasca, R., Gattei, V., Oscier, D., Bertoni, F., Mullighan, C., Foa, R., Pasqualucci, L., Rabadan, R., Dalla-Favera, R., & Gaidano, G. (2011). Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation Journal of Experimental Medicine DOI: 10.1084/jem.20110921
Fabbri, G., Rasi, S., Rossi, D., Trifonov, V., Khiabanian, H., Ma, J., Grunn, A., Fangazio, M., Capello, D., Monti, S., Cresta, S., Gargiulo, E., Forconi, F., Guarini, A., Arcaini, L., Paulli, M., Laurenti, L., Larocca, L., Marasca, R., Gattei, V., Oscier, D., Bertoni, F., Mullighan, C., Foa, R., Pasqualucci, L., Rabadan, R., Dalla-Favera, R., & Gaidano, G. (2011). Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation Journal of Experimental Medicine DOI: 10.1084/jem.20110921