The relationship between radioactivity, leukemias and lymphomas
Ohayo gozaimasu! Like many people this weekend, I have been following the twin earthquake and tsunami disaster in Northern Japan with a great sense of sadness after hearing that thousnads of people have died. If you haven’t already seen it, pictures of Japan before and after the tsunami devastation are an astonishing testament to the power of nature. Coming so soon after the harrowing pictures of the massacres in Libya, it certainly feels like a violent world at the moment.
It is interesting to follow the news on Twitter in real time, generally much more useful (bar some hype and silliness from some people) than CNN and other News outlets who struggled to get to grips with timely information. I was, however, disheartened to see that the engineers at Fukushima had no choice but to resort to venting the gases and also pumping in borax and sea water into the reactor chamber. They needed to do this to try and cool the reactors down, reducing pressure, thus try to prevent explosions (and noxious radioactivity escaping into the atmosphere) or even worse, a core meltdown.
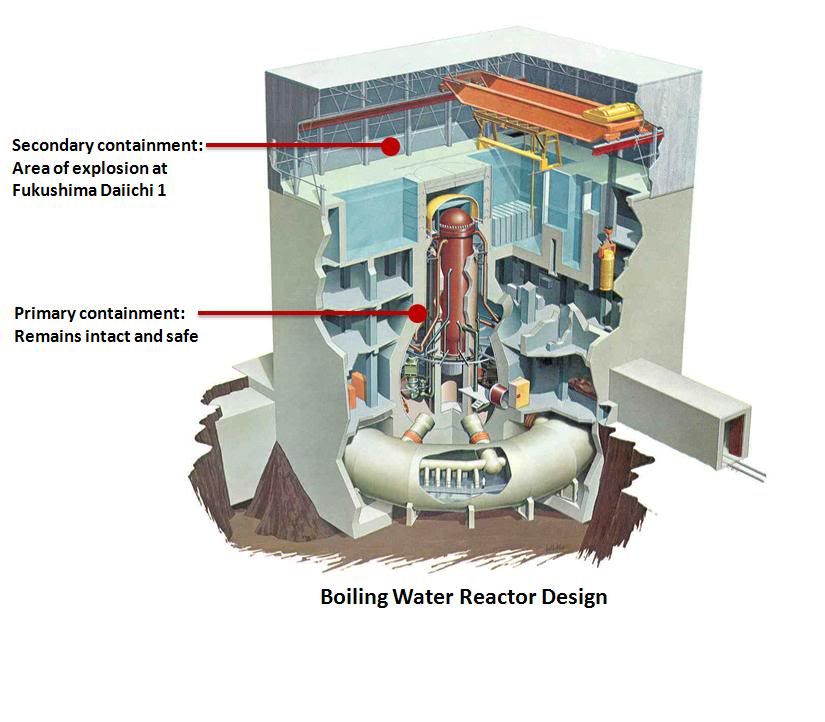
One source of accurate information was the Nuclear Energy Institute, who provided regular, factual updates on their website and pictures to explain what was going on with the Fukushima Boiling Water Reactors or BWR in short (you can see where the NY Times and others got their pictures from, for example):
Social Media is useful for collating information from local people in Japan, such as this brief but alarming video from Fukushima courtesy of Japanese tv of one of the explosions at the nuclear plant:
There was some great commentary on Twitter from nuclear professionals such as Arclight, who spent most of his weekend monitoring the situation and answering anxious people’s questions about what was going on. In times like these, accurate information rather than speculation is more more helpful and reassuring.
It is, however, decay heat that creates challenges, ie heat that has been stored in the rods and is expelled rapidly if they are not cooled sufficiently or exposed to air, thereby creating hot steam and pressure. The NY Times has an excellent simplified graphic that explains how nuclear reactors work for those interested.
The problem is that high levels of radioactivity in the sea or air is never a good thing for life around it. Years ago, there was a hugh debate (that’s an understatement) about the increased incidence of leukaemia and lymphomas in children around the Sellafield nuclear plant in Cumbria, UK, with different groups arguing for and against it (see references below as a snapshot).
The argument about cause and effect doesn’t seem to have been settled either way judging from various epidemiology studies and intense papers/letters to journals such as the BMJ over the past few decades. Still, I’m sure most of us would probably agree that exposure to direct prolonged radiation from the sea or air is, intuitively, probably not a good thing in the long run. Strontium, for example, one of the chemicals produced as a by product in nuclear reactors, tends to build up in teeth and bones, and may well be an irritant that can cause mutations over time.
What is clear, is that once sea water is pumped into a reactor, it is essentially dead and can never be used again, necessitating a massive clean up operation that may well take years to effect. If anyone can do it efficiently and effectively, it is probably the Japanese with their superb engineering and sadly, experience from the Hiroshima clean up after the Second World War.
This morning, the availability of clean air, food and water took on an entirely different meaning. In the meantime, my thoughts are sincerely with the Japanese people in their hour of need.
References:
![]() Gardner, M., Snee, M., Hall, A., Powell, C., Downes, S., & Terrell, J. (1990). Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ, 300 (6722), 423-429 DOI: 10.1136/bmj.300.6722.423
Gardner, M., Snee, M., Hall, A., Powell, C., Downes, S., & Terrell, J. (1990). Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ, 300 (6722), 423-429 DOI: 10.1136/bmj.300.6722.423
Gardner MJ, Hall AJ, Snee MP, Downes S, Powell CA, & Terrell JD (1990). Methods and basic data of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ (Clinical research ed.), 300 (6722), 429-34 PMID: 2107893
Roman E, Watson A, Beral V, Buckle S, Bull D, Baker K, Ryder H, & Barton C (1993). Case-control study of leukaemia and non-Hodgkin’s lymphoma among children aged 0-4 years living in west Berkshire and north Hampshire health districts. BMJ (Clinical research ed.), 306 (6878), 615-21 PMID: 8461811
Watson GM (1991). Leukaemia and paternal radiation exposure. The Medical journal of Australia, 154 (7), 483-7 PMID: 2005848
Draper GJ, Little MP, Sorahan T, Kinlen LJ, Bunch KJ, Conquest AJ, Kendall GM, Kneale GW, Lancashire RJ, Muirhead CR, O’Connor CM, & Vincent TJ (1997). Cancer in the offspring of radiation workers: a record linkage study. BMJ (Clinical research ed.), 315 (7117), 1181-8 PMID: 9393219
 This year, there was no new data or ideas expounded in the sessions I chose, they were basically a run through of the old data with a couple of slides at the end listing a few compounds that are in development. The pipeline in for hematologic malignancies is gradually gaining more attention from Pharma, which is great news, although they are probably a lot less crowded than the main solid tumours.
This year, there was no new data or ideas expounded in the sessions I chose, they were basically a run through of the old data with a couple of slides at the end listing a few compounds that are in development. The pipeline in for hematologic malignancies is gradually gaining more attention from Pharma, which is great news, although they are probably a lot less crowded than the main solid tumours.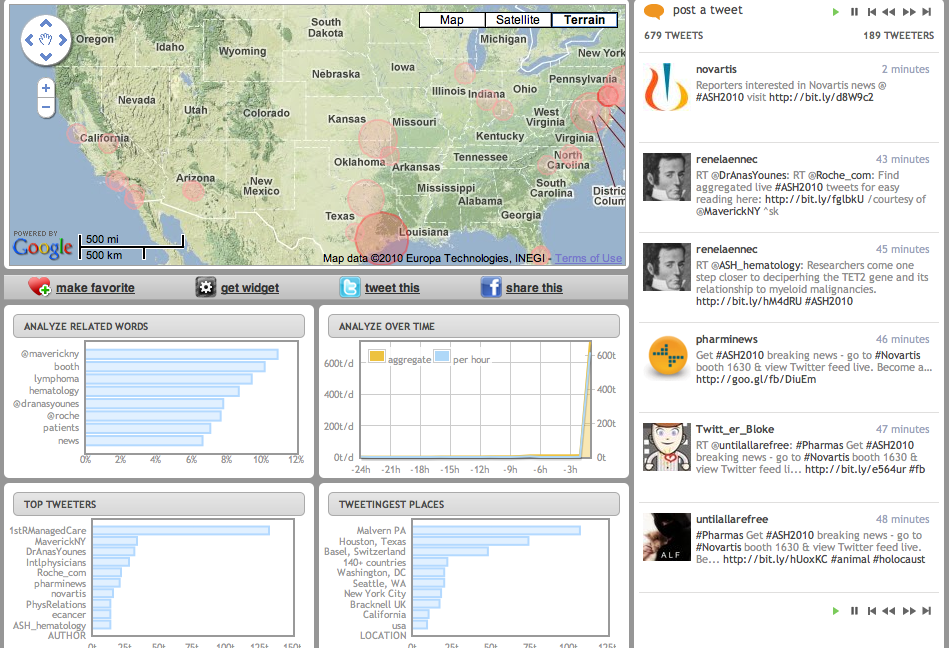
 Later in the poster session, I tracked down Michael Becker (
Later in the poster session, I tracked down Michael Becker ( If you’re not watching Semafore and another PI3-kinase company, Calistoga, that I mentioned in my What’s Hot at ASH 2010 post, then check them out as they both have novel and interesting compounds that may be eventually licensed by pharma or biotech companies. You can also read more about the background behind PI3-kinase as a valid cancer target
If you’re not watching Semafore and another PI3-kinase company, Calistoga, that I mentioned in my What’s Hot at ASH 2010 post, then check them out as they both have novel and interesting compounds that may be eventually licensed by pharma or biotech companies. You can also read more about the background behind PI3-kinase as a valid cancer target 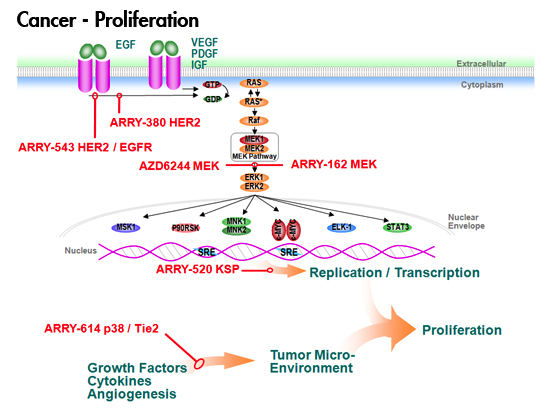
 Dr Younes is very active in social media on
Dr Younes is very active in social media on