Update on advanced prostate cancer including enzalutamide (MDV3100) and abiraterone
This month has brought a flurry of regulatory activity in the prostate cancer landscape with several companies seeing noticeable action in the new product development area:
- Medivation/Astellas filed enzalutamide (MDV3100) with the FDA in metastatic castration resistant prostate cancer (mCRPC) in the post chemotherapy setting based on the AFFIRM trial
- The UK NICE approved abiraterone (Zytiga) in the same setting (except in Scotland), but rejected cabazitaxel (Jevtana)
For this specific post, I want to concentrate on the new trends in advanced prostate cancer, as last weekend I attended the 2012 American Urological Association (AUA) annual meeting, focusing on the basic research sessions hosted by the Society of Basic Urological Research (SBUR) and Society of Urologic Oncology (SUO). It’s hard to ignore the clinical data though, with so much activity in going on in advanced prostate cancer!
While at the oral and poster sessions, I enjoyed informal chats with urologists and researchers. Many of the urologists I spoke to at AUA were very excited about enzalutamide, certainly much more enthusiastic than in the last two years when we saw the arrival of sipuleucel-T (Provenge) and abiraterone (Zytiga) in the pre- and post- chemo settings respectively. The reasons for this were varied, depending on the respondents, ranging from understanding the mechanism of action (MOA) clearly to ease of logistics to lack of concomitant steroid therapy. The fact that the initial overall survival of 4.8 months for enzalutamide in the post-chemo setting, which hints at a more potent and effective therapy, probably helped as well.
With that in mind, I spoke with Dr Neal Shore, one of the AFFIRM trialists about his perspective of the impact of enzalutamide on his patients. Firstly, we discussed the expectations for patients who have received chemotherapy:
“Patients who typically have completed a course of docetaxel have historically a 6-12 month life expectancy depending upon… on how symptomatic or asymptomatic they are.”
Secondly, what was the impact of treatment with enzalutamide?
“In the treatment arm, those who received enzalatumide, they lived at the median 4.8 months longer than patients who received the control placebo.
So in fact their survival extended beyond 18 months. I told you that normally this patient population has a 6-12 month survival expectancy range, that to me is dramatic.
It’s a dramatic life prolongation effect.”
Emphasis mine.
One of the things that makes enzalutamide exciting for me is the ability to target splice variants. This may explain why the agent has slightly better efficacy in the post chemo setting than abiraterone, which demonstrated a 3.9 month overall survival advantage over placebo when it was initially approved. This figure has since improved to 4.7 months with more mature data, suggesting that the initial 4.8 months improvement we see with enzalutamide may also potentially improve further with time.
Previously, I discussed the splice variants with Dr David Hung, the Medivation CEO:
Dr David Hung on Splice Variants:
Another area where we may see changes in mCRPC is sequencing, combinations and different trial designs. While it seems logical that good old fashioned sequencing, as with ADTs in earlier disease, will prolong time to disease progression by managing PSA levels, it should be noted that I don’t think any of those agents have actually shown a significant improvement in overall survival in patients. Unlike oncology, urology tends to have looser endpoints and this is something that we may well see changes in going forward as more rigour is applied in the clinical trial setting.
Combination therapy is something that I think we will also see more of in the future if we are to see real shifts in meaningful outcomes. As Charles Sawyers noted in a previous interview, shutting down the AR pathway more comprehensively with dual inhibition and ‘big guns’ makes solid scientific sense. This doesn’t just mean the obvious though, such as determining whether dual upstream and downstream AR inhibition with enzalutamide plus abiraterone versus either alone would work, but also targeting cross-talk and adaptive resistance pathways such as AR inhibition plus a PI3K inhibitor. Some of these trials are already underway, at least in phase II to see what the safety and efficacy signals looks like.
The arrival of multiple new therapies that change the standard of care also means that once the current crop of new drugs have been approved (abiraterone, enzalutamide and alpharadin) it becomes more difficult to conduct placebo controlled trials with OS any more, as Dr Shore astutely observed:
Dr Shore’s point about surrogate markers of survival in advanced cancer is a highly relevant one given the increasing level of competition in this tumour type.
In the pre-chemotherapy setting, both abiraterone (302 trial) and enzalatumide (PREVAIL) appear to have progression-free survival (PFS) and OS as co-primary endpoints. It will be interesting to see how the FDA react if only PFS is significant while OS is not, as many commentators suspect from the rather vague and woolly press release that J&J put out for abiraterone. I don’t know what was agreed beforehand with the FDA regarding the study design, but I can see an interesting and highly charged ODAC ahead here as well as much speculation prior to the ASCO presentation of the data in the prostate cancer oral session next month.
OS is a clear, but challenging measure because ultimately, as Dr Shore noted, the endpoint is death. Remember, recent breast cancer trials discussed here on PSB achieved a significant OS of 6 months or more, precisely because crossover wasn’t allowed. These trials had an active arm as a comparator, not placebo, though. We don’t yet know if patients in the placebo arm who relapsed were allowed to crossover to abiraterone early (ie before the IDMC recommendation). If they did, then OS was doomed from the start and the trial design itself was flawed, but we will have to wait for the presentation to see before jumping to conclusions.
I really hope that’s not the case here, because once crossover does occur, it is very hard statistically, to sort out a true survival signal. If the placebo patients didn’t crossover before the IDMC recommended study stoppage, then it’s hard to see why OS wasn’t met (if truly the case) unless the patients had long term compliance and adherence problems as a result of the concomitant steroid therapy. I do find it hard to believe, however, that an IDMC would stop a trial early if a primary registration endpoint was not met, for that would be akin to regulatory hari-kiri!
Overall survival has long been the standard of care in advanced prostate cancer and several drugs have either received approval or will receive approval as a result of meeting that high hurdle. Future entrants will find it very hard to ethically justify using placebo in the comparator arm and will likely need to be compared to sipuleucel-T, docetaxel, cabazitaxel or abiraterone as the currently approved new standards of care and potentially other options will be considered that are seeking approval such alpharadin and enzalutamide in the near future.
This improvement in care raises the bar for companies considering advanced prostate cancer in several ways:
- Increased costs (active comparator arm, prolongation of treatment)
- Increased time to market (OS takes longer than PFS)
In the end, though, for men with prostate cancer it’s largely all good news as new therapies that clearly prolong life become available and make a difference to their lives, not just in terms of more time with their families, but also in terms of improved quality of life and symptom management.
 At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.
At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.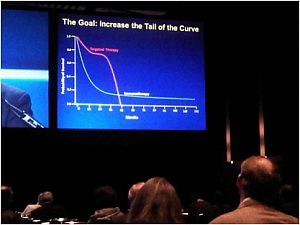 The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.
The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.