The role of the Androgen Receptor in breast cancer
This week I have been in Orlando for the American Association for Cancer Research (AACR) Special Conference on prostate cancer chaired by Drs Arul Chinnaiyan (U. of Michigan) and Charles Sawyers (MSKCC). It was a superb meeting, probably one of the best I’ve attended since the PI3K meeting that AACR hosted in February last year. I wrote nearly half a Moleskine of notes that vaguely resemble chicken scratch – there were so many good talks that stimulated new ideas and explained a few scientific things I also didn’t know too well. Learning is a continuous lifetime experience, after all.
During the meeting, I had a nice correspondence with one of our regular blog readers, the thoughtful Biomaven. Peter mentioned some data on the androgen receptor (AR) as a potential target in breast cancer following Medivation’s recent conference call. It’s an interesting topic and one well worth discussing. Here’s a map of the AR pathway for reference:
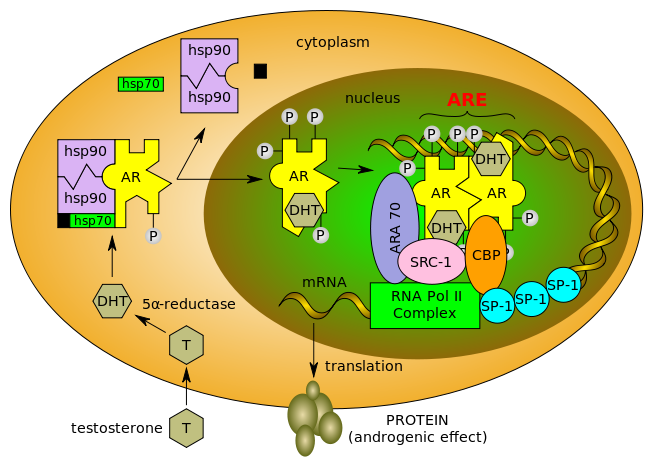
Source: wikipedia
The AR is not something one naturally and immediately thinks of in women, since testosterone is usually considered a manly thing. That said, it is present in women in both normal breast epithelial cells and ~70% to 90% of invasive breast cancers.
Until recently, the link, however between AR status and breast cancer survival is uncertain and perhaps a little controversial, but Hu et al., (2011) looked at the association between the AR status and breast cancer survival in the Nurses’ Health Study (NHS) – see references at the end for the link to the article.
What was the study about?
According to the authors:
“The NHS is a prospective cohort study established in 1976 when 121,700 female registered nurses from across the United States, aged 30 to 55 years, completed a mailed questionnaire on factors that influence women’s health.
Follow-up questionnaires have since been sent out every 2 years to the NHS participants to update exposure information and ascertain nonfatal incident diseases. Follow- up rate from 1976 to December 2007 is 98.9% in our study.”
Not to be confused with an population/epidemiology study from the NHS (National Health Service) in the UK! The main goal of this study was to:
“… determine the association of AR status with survival outcomes adjusting for covariates.”
What did the research find?
Out of all the breast cancers followed (n=1467), 78.7% were AR+. Additionally, amongst the ER+ patients (n=1,164), 88% were AR+:
“AR positivity was associated with a significant reduction in breast cancer mortality (HR, 0.68; 95% CI, 0.47–0.99) and overall mortality (HR, 0.70; 95% CI, 0.53–0.91) after adjustment for covariates.”
The situation was very different in women who were ER- (n=303) though:
“42.9% were AR-. There was a nonsignificant association between AR status and breast cancer death (HR, 1.59; 95% CI, 0.94–2.68).”
In other words, AR+ confers a better prognosis in ER+ breast cancer.
Now, the relevance of all this research is potentially important when considering possible mechanisms of resistance to aromatase inhibitor (AI) therapy in ER+ breast cancer. Recall that one mechanism of resistance to AI treatment is mTOR, which is why the BOLERO2 trial with an AI (exemestane) plus an mTOR (everolimus) in the relapsed setting did so well in ER+ women. Not all of the women in the trial responded to the treatment though, suggesting that other factors may play a role in acquired or adaptive resistance.
What is the importance of AR to therapies for breast cancer?
Normally, knowing whether a particular situation has a better or worse outcome isn’t particularly helpful for patients, since it doesn’t predict which therapy might be more appropriate. However, there is some other AR and breast cancer research from Cochrane et al., (2011) which was presented to the Endocrine Society Peter referred to that tells us a bit more of the AR story:
“We postulate that ER+ breast cancers that fail to respond or become resistant to current endocrine therapies (tamoxifen or AI) may do so because they have switched from growth controlled by estradiol (E2) and ER to growth controlled by liganded AR.
We therefore sought to determine if blocking AR activity could serve as a therapeutic intervention for such tumors.”
What did they do?
Cochrane et al, (2011) stated that:
“We used breast cancer cells that express ER and AR such as MCF7 cells and a cell line that we recently isolated that contains more AR than ER.
Our data indicate that although DHT does slightly inhibit E2-mediated proliferation, DHT alone is proliferative in cells such as MCF7 with both ER and AR, and is even more proliferative than E2 when AR is more abundant than ER.”
What did the results show?
The results were a) interesting and b) a little surprising:
“We found that while both the anti-androgen bicalutamide and the triple acting, non-steroidal, AR antagonist MDV3100 block DHT and R1881-mediated proliferation of breast cancer cells, we made the novel observation that MDV3100, but not bicalutamide, inhibits E2-mediated proliferation of breast cancer cells.”
These results led the authors to conclude that:
“Anti-androgens, such as MDV3100, may be particularly useful to treat patients whose tumors fail to respond to traditional endocrine therapy despite being ER+, or who have ER-/AR+ tumors.”
Not surprisingly, Medivation announced on their recent conference call this month that they will be seeking to explore this phenomenon in clinical trials. I think this is a logical and exciting development that is well worth a shot on goal. We know that not all the women in the BOLERO2 trial responded to exemestane and everolimus, so other mechanisms must be at play here. This is certainly worth exploring.
The question with the study design of me for me though, is patient selection. How do we determine which women whose initial AI therapy leads to relapse should go onto an mTORor an AR antagonist? I’m guessing that maybe biopsies will be part of the answer.
In conclusion…
On the positive side, it would be pretty cool if we could uncover two mechanisms of resistance to AI therapy in ER+ breast cancer and have some viable therapies to offer women once relapse or acquired resistance sets in. It would start to offer a) hope and b) potentially prolong outcomes further as we determine ways to shut down the various escape routes and signaling pathways. If the concept works, given that up to 30% of women with ER+ breast cancer may have AR+ signaling, then it would also be good news for Medivation and Astellas with MDV3100’s potential upside.
References:
![]() Hu, R., Dawood, S., Holmes, M., Collins, L., Schnitt, S., Cole, K., Marotti, J., Hankinson, S., Colditz, G., & Tamimi, R. (2011). Androgen Receptor Expression and Breast Cancer Survival in Postmenopausal Women Clinical Cancer Research, 17 (7), 1867-1874 DOI: 10.1158/1078-0432.CCR-10-2021
Hu, R., Dawood, S., Holmes, M., Collins, L., Schnitt, S., Cole, K., Marotti, J., Hankinson, S., Colditz, G., & Tamimi, R. (2011). Androgen Receptor Expression and Breast Cancer Survival in Postmenopausal Women Clinical Cancer Research, 17 (7), 1867-1874 DOI: 10.1158/1078-0432.CCR-10-2021
4 Responses to “The role of the Androgen Receptor in breast cancer”
Interesting though there is also data from Ana Gonzales here MDACC showing AR is prognostic in breast cancer (high = better outcome) and is associated with PI3K mutations. Article in CCR –> http://clincancerres.aacrjournals.org/content/15/7/2472.full
Hi Angela, Actually that would be very consistent since Hu et al., were saying that high AR+ had a better outcome in ER+ breast cancer. The PI3K mutations is interesting and not surprising, since Sawyers and Rosen published a paper in Cancer Cell last year showing that inhibiting AR activated PI3K and vice versa in prostate cancer, so it would not surprise me to see a similar effect in breast cancer. It would certainly speak to a very logical trial of MDV3100 + a PI3Ki in breast in women with high AR+ expression, rather than MDV3100 or PI3K alone. Nice paper, many thanks!
Sally, This seems like such a fantastic new avenue of research.Thank you for writing this up. I wasn’t aware of these findings.
Really the great post, which have worth.. I appreciate this post.
Comments are closed.