ASCO 2013 Highlights PD-1 and PD-L1 immunotherapy
One of the hot topics at this year’s annual ASCO meeting is clearly going to be PD-1 and PD-L1 immunotherapies, following on from the success of BMS’ PD-1 agent highlighted in my ASCO video last year. By now, we know that it has a generic name, nivolumab, and is being studied in combination with ipilimumab (Yervoy) in metastatic melanoma. You can find the many nivolumab abstracts here.
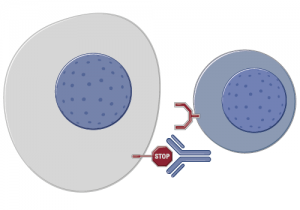
Source: Roche
Interestingly, BMS also now has competition from other big pharma companies, including Roche’s PD-L1 antibody, MPDL3280A, and Merck’s lambrolizumab. Roche appear to be developing a companion diagnostic, which could mean the responses are different for PD-L1 positive and negative patients. At least, that would be an intuitive conclusion and a potentially good way to preselect patients who are most likely to respond to the therapy.
In addition, the FDA have already picked up on this class of agents, conferring the almost ubiquitous Breakthrough Therapy status on some of them already, so solid data in the next year or so could mean a fast track to market strategy is possible in this category.
In the battle of the abstracts and sheer depth of data in the PD-1/PD-L1 segment, you can see that Merck are already the poor cousins to BMS and Roche in execution. Just one? That’s pitiful! Never a good sign.
What factors need to be considered in looking at the immunotherapy data?
Last night I was searching the abstracts on my iPhone after the local broadband inconveniently went down and also watched the conversations on Twitter, usually a fun experience fishing for and discussing the diamonds in the rough. A couple of things struck me, however, around the immunotherapy data mining and chatter:
Firstly, there’s way too much focus on ORR (overall response rate) and the minutiae of the differences between the different PD-1/PD-L1 agents. It’s far too early to tell much, as we all know that what matters are the randomized phase III trials, trial design and patient selection (specific, catch-all etc). These can all have a huge impact on the final outcomes in large scale randomised studies.
Secondly, ORR is a measure of disease control – it tells us how much shrinkage there is going on at the time of measurement and is based on RECIST. This is partly a hangover form old chemo days, and partly a lack of available biomarkers of response. Let’s not also forget that immunotherapies usually have a delayed effect and while waterfall plots at six months or so are useful, they don’t tell us what the long term effects will be. How durable will the responses be beyond 6-8 months? Is there adaptive resistance developing? What sort of logical combinations and sequencing options can be considered? So many questions to which we have no answers yet.
Thirdly, be very careful when interpreting the abstract data for ORR – sometimes the data is given for all the patients, irrespective of whether they responded or not, and sometimes it is given as a percentage of the patients who actually had some sort of response. You need to compare apples with apples when looking across studies or the conclusions drawn can end up being a little off.
Fourthly, I don’t think ORR is the ideal endpoint. So what? What really matters is how long do patients live, do they feel better (or worse) and will they have a better quality of life as a result of taking the medication? Other obvious but important questions we need to evaluate going forward include:
- How much of a prolonged effect with PD-1/PD-L1 immunotherapies have over 5 years?
- What will be their effect on subsequent therapies?
- Will they boost or hinder sequencing and in which tumour types?
- Is there a biomarker of response?
- Is a diagnostic necessary?
Fifthly, combination studies are nice if they lead to improved outcomes, but at what cost will this be achieved? By this, I mean both in terms of safety (remember ipi and vemurafenib were thought to be a logical combo in melanoma until the unexpected AEs scuppered that concept) and also cumulative cost of treatment. None of the new oncology therapies can or will be considered inexpensive these days, especially when the benefit might be measured in only a few months or less.
Overall:
I’m really looking forward to these presentations on PD-1/PD-L1 and will write about them in more detail at the meeting. It continues to be an exciting area in oncology, as long as the results live up to the expectations. It’s still unclear which tumour types will benefit most and what the durability will be. Right now, I have more questions than answers, but as a concept it’s definitely one well worth watching over the next couple of years.
2 Responses to “ASCO 2013 Highlights PD-1 and PD-L1 immunotherapy”
Hi Sarie, I was intrigued by your comment on Merck being the poor cousing when it comes to depth of data in their abstracts. They are holding an analyst day this Sunday, do you have any thoughts as to what to expect from it?
With regard to Ipi/Zelboraf combo, the sequencing trial is still ongoing in BRAF mutant melanoma patients, first Ipi, and then Zelboraf. Do you expect similar side effects here?
Thank you.
Hi Juan,
Re: poor cousin – both BMS and Roche have multiple abstracts and studies for their PD-1/PD-L1 antibodies at ASCO, while Merck have just a single abstract. In terms of output, they’re therefore a bit behind. Have no idea what’s happening with the analyst day as bloggers are not usually invited 😉
WRT ipi+vem, I’m hoping that sequencing will be a) more effective and b) less toxic than the combination, but we will have to wait and see what the data says.
Comments are closed.