A couple of interesting developments have emerged over the last week with AKT and MEK inhibitors, specifically Merck’s MK-2206 and AstraZeneca/Array’s AZD6244, that are well worth discussing.
- At the ECCO/EMCC meeting in Stockholm last Tuesday, Johann De Bono discussed the combination data for MK-2206 and AZD6244 in KRAS driven colorectal cancer.
- Later the same week, Array Biopharma announced the initial results from a randomized phase II placebo-controlled study that compared the efficacy of selumetinib (AZD6244/ARRY-886) in combination with docetaxel compared to docetaxel alone in the second-line treatment of patients (n=87) with KRAS-mutant, locally advanced or metastatic non-small cell lung cancer (NSCLC).
Now, to be clear, I like the concept of AKT and MEK inhibitors, especially in select combinations, but the key thing here is the right combinations in the right context.
Let’s take a look at the lung cancer KRAS data first. One of the challenges I have with this approach, is that we’ve know for a while that BRAF and KRAS driven cancers behave rather differently according to Wee et al., (2009):
“Previous studies have found that whereas BRAF mutant cancers are highly sensitive to MEK inhibition, RAS mutant cancers exhibit a more variable response.”
Variable response is not an encouraging phrase when planning clinical trials!
Let’s take a look at the pathway itself:
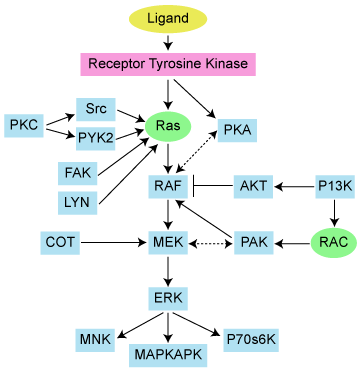
We can immediately see that MEK is downstream of RAS, meaning that even if we target MEK, unfortunately RAS and KRAS is still largely untouched upstream. This is important to remember when considering the actual results later.
The other key factor to consider is what are the adaptive resistance pathways that might evolve as a result of treatment with a MEK inhibitor? In an ideal world, logical combinations would be tested that target both the primary driving mutation or aberration, as well as the adaptive resistance, to try and shut down the pathway more completely than targeting either alone. Another key question that needs to be addressed is what is driving the KRAS aberrant activity in the first place?
We’ve discussed MEK numerous times here on PSB, but the Wee et al., (2009) MEK paper stands out in particular. They identified a critical resistance pathway to MEK inhibition, namely PI3K. Although we discussed this originally in the context of BRAF driven tumours such as melanoma, it is well worth discussing again here in regards to KRAS driven tumours given a MEK inhibitor is being tested.
They observed that:
“Activating mutations in PIK3CA reduce the sensitivity to MEK inhibition, whereas PTEN mutations seem to cause complete resistance.”
It isn’t clear from the Array press release whether any of the patients with NSCLC exhibited PIK3CA mutations or loss of PTEN, but they definiely do occur in this disease. It will be interesting to see of more meta data is available at the forthcoming AACR Molecular Targets meeting next month.
I’m not a big fan of chemotherapy plus a single targeted agent, because as you can see from the evidence above, the pathway is not being shut down by one targeted agent and resistance is not being addressed at all. The chances of such a combination working (by that I mean increasing overall survival), I think would be fairly low.
According to the press release, the study did not see a significant improvement in overall survival (OS) but did show an encouraging response in the form of progression free survival (PFS):
“The key secondary endpoints of progression-free survival, objective response rate, and alive and progression-free at 6 months were all demonstrated with statistical significance, showing improvement in favor of selumetinib in combination with docetaxel versus docetaxel alone.”
Indeed, at the recent AACR and ASCO meetings, there was also some encouraging early signs from Genentech’s PI3K inhibitor, GDC-0941, as a single targeted agent with chemotherapy in NSCLC (a very small early trial), albeit not KRAS specific, but defined more broadly by squamous and non-squamous histology. Thus, all is not lost with the MEK agent yet – if we combined MEK and PI3K inhibitors in NSCLC patients previously treated with chemotherapy, we might have a better chance of succeeding and shutting down the pathway, based on evidence offered from Wee et al’s preclinical research:
“At the molecular level, the dual inhibition of both pathways seems to be required for complete inhibition of the downstream mammalian target of rapamycin effector pathway and results in the induction of cell death.”
As a result, they went onto to suggest a logical treatment approach:
“Our study provides molecular insights that help explain the heterogeneous response of KRAS mutant cancers to MEK pathway inhibition and presents a strong rationale for the clinical testing of combination MEK and PI3K targeted therapies.”
Of course, clinical trials like this always progress incrementally, such that we test a MEK or a PI3K inhibitor alone to determine safety and efficacy activity, then perhaps in combination, which requires another phase I dose finding study to determine the ideal dosages and whether they are too toxic or not combined.
So while either single agent targeted therapy with chemotherapy in and of itself is not a win, there are signs that combining the two may be more appropriate. I would still want to know what is driving the KRAS activity though, given MEK and PI3K are downstream of it. It is entirely possible that a third agent would be needed to shut down the pathway more completely in that patient subset.
At ECCO, De Bono (Royal Marsden) discussed the combination of AstraZeneca’s MEK inhibitor (AZD6244) and Merck’s AKT inhibitor (MK-2206) in RAS mutant colorectal (CRC) and lung (NSCLC) cancers. The results here were not a big win in the former, with 8/15 patients showing no antitumour activity to date.
There are several things we can conclude from the initial data:
- If we have the right combination for the right target in the right patient subset, then the therapeutic index of the agents is lacking and we need better drugs
- Are the targets (AKT and MEK) critical?
- Is something else driving the KRAS activity (see below)*?
- Are we shutting down the adaptive resistance pathways (escape routes?)
- Which patient subsets are most likely to respond and how do we best characterise them (ie need more biomarker data)?
And so on… there are always more questions than answers sometimes.
* Note: This situation could well be similar to BRAF in malignant melanoma, where it is the V600E mutation that is driving the BRAF activity, thus specifically targeting ithe mutation rather than the kinase will have a greater clinical effect than targeting BRAF broadly. In this case, if we really believe KRAS is critical to the lung or colorectal tumour’s survival, then we need to figure out what is driving it before progress is made. Frank McCormick’s elegantly simple wac-a-mole concept for pathway inhibition is very apt here!
No doubt we will see more detailed data and an update soon, perhaps even at the forthcoming AACR Molecular Targets meeting next month.
References:
 Wee, S., Jagani, Z., Xiang, K., Loo, A., Dorsch, M., Yao, Y., Sellers, W., Lengauer, C., & Stegmeier, F. (2009). PI3K Pathway Activation Mediates Resistance to MEK Inhibitors in KRAS Mutant Cancers Cancer Research, 69 (10), 4286-4293 DOI: 10.1158/0008-5472.CAN-08-4765
Wee, S., Jagani, Z., Xiang, K., Loo, A., Dorsch, M., Yao, Y., Sellers, W., Lengauer, C., & Stegmeier, F. (2009). PI3K Pathway Activation Mediates Resistance to MEK Inhibitors in KRAS Mutant Cancers Cancer Research, 69 (10), 4286-4293 DOI: 10.1158/0008-5472.CAN-08-4765
 In other words, it’s too expensive and the NHS doesn’t want to pay the £80K ($120K) sticker price. This news is no great surprise given the cost-benefit ratio when considering that there is no way to tell who might benefit most from treatment upfront.
In other words, it’s too expensive and the NHS doesn’t want to pay the £80K ($120K) sticker price. This news is no great surprise given the cost-benefit ratio when considering that there is no way to tell who might benefit most from treatment upfront.