Last week there was lot of excitement and interest surrounding the blog post on Roche/Plexxikon's data on PLX4032 in metastatic melanoma published in the New England Journal of Medicine. A number of the discussions on Twitter and email centred around what is causing resistance to the BRAF inhibitor?
If we take a look at the BRAF pathway alone, we would get a sense of the flow from the PDGF ligand through RAS, RAF and MAPK, which essentially drives angiogenesis and proliferation, like this 2004 review article:

Source: Nature Reviews Cancer
However, what this sort of simple diagrammatic picture doesn't tell us though, is where cross-talk or feedback loops might interfere with the inhibition to enable cell signalling to continue, thereby guaranteeing the tumour's continued survival despite our efforts to shut it down.
Another way of looking at the problem is to consider how pathways related to RAS signalling might possibly interact with the process, like this:
 Source: Reaction Biology
Source: Reaction Biology
When we look at the cell signalling processes this way, we can see that inhibiting PI3-kinase or AKT at the same time as RAF might turn out to be a useful approach.
It was therefore with great interest while browsing my RSS cancer feeds that I spotted a paper by Shao and Aplin in the current Cancer Research journal entitled: "Akt3-Mediated Resistance to Apoptosis in B-RAF–Targeted Melanoma Cells" (see journal link below).
If we use a BRAF inhibitor such as PLX4032 in advanced melanoma, we are effectively trying to kill the cancer cells by inducing apoptosis, or programmed cell death, thereby reducing the tumour growth and proliferation. However, as the researchers put it very succinctly:
"Melanoma cells are highly resistant to anoikis, a form of apoptosis induced in nonadherent/inappropriate adhesion conditions. Depleting B-RAF or the prosurvival Bcl-2 family protein Mcl-1 renders mutant B-RAF melanoma cells susceptible to anoikis."
They therefore began looking at different approaches to dealing with anoikis using both inhibitors and RNA interference.
These concepts may help us better understand how mutant B-RAF protects melanoma cells from apoptosis, thereby providing insight into possible resistance mechanisms to B-RAF inhibitors by developing new mutations in AKT. It also paves the way for future therapeutic strategies, either in combination, or in sequence, with a V600E BRAF inhibitor such as PLX4032 and an Akt inhibitor. I'm also wondering what effect a PI3-kinase inhibitor might have, but clearly adding an Akt inhibitor to the mix may well be worth a try in the first instance.
There are a number of AKT inhibitors in development in various companies pipelines. This is where the challenges and hurdles begin if a company doesn't have a relevant inhibitor because traditional R&D focuses on developing one's own pipeline with or without the current standard of care rather than cross-development with other companies with novel combination treatments unless a partnership, particularly with a small biotech, is specifically sought out.
As far as I know, Roche/Genentech or Plexxikon don't have an AKT in their pipelines, but there are some currently in early clinical development with other companies. The PI3K-mTOR-AKT pathway has been discussed in more detail in previous blog posts.
The most interesting and promising compound in this class is probably Merck's MK-2206, currently in phase II development for a number of different tumour types.
Other Akt inhibitors in R&D include:
- Keryx: perifosine in phase II development for myeloma and colorectal cancer
- Rexahn: Archexin in phase II trials in pancreatic cancer
- Nerium: oleandrin in phase I development for metastatic renal and colorectal cancers
- GSK: GSK2141795 and GSK21110183 are both in phase I trials for either hematologic malignancies or solid tumours
GSK had an earlier Akt inhibitor in phase I, GSK690693, but I believe it may have been terminated due to problems with hyperglycaemia, a common problem associated with PI3K-IGF-1R feedback. This problem has since been addressed and managed in other trials associated with these agents by the simple addition of metformin in the protocol. GSK now appear to be focusing on the follow-on compounds instead.
All in all, it's interesting to see how our knowledge of the biochemical and molecular pathways helps us better understand how cancer works and how we can use the data to devise improved strategies for tackling melanoma in the future. I'll be watching how this field develops with close interest.
 Dibb, N., Dilworth, S., & Mol, C. (2004). Opinion: Switching on kinases: oncogenic activation of BRAF and the PDGFR family Nature Reviews Cancer, 4 (9), 718-727 DOI: 10.1038/nrc1434
Dibb, N., Dilworth, S., & Mol, C. (2004). Opinion: Switching on kinases: oncogenic activation of BRAF and the PDGFR family Nature Reviews Cancer, 4 (9), 718-727 DOI: 10.1038/nrc1434
Shao, Y., & Aplin, A. (2010). Akt3-Mediated Resistance to Apoptosis in B-RAF-Targeted Melanoma Cells Cancer Research, 70 (16), 6670-6681 DOI: 10.1158/0008-5472.CAN-09-4471
Crouthamel, M., Kahana, J., Korenchuk, S., Zhang, S., Sundaresan, G., Eberwein, D., Brown, K., & Kumar, R. (2009). Mechanism and Management of AKT Inhibitor-Induced Hyperglycemia Clinical Cancer Research, 15 (1), 217-225 DOI: 10.1158/1078-0432.CCR-08-1253

 At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.
At first view, I was impressed by the enormous organization and very large number of participants, which was at least as important as the ASCO annual meeting. However, as a clinician, I only recognized very few familiar faces as the large majority of attendance included basic and translational scientists, as well as representatives of pharma.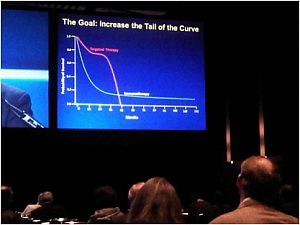 The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.
The goal is to increase the tail of the curve in the photograph in the right. The approval of ipilimumab in the treatment of metastatic melanoma has inaugurated the new era of anti-cancer immune therapies.
![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=a00cea0a-1b38-4a54-8304-f0823641685a)