Zoledronic acid did not improve disease free survival in early breast cancer
Wow, every now and then, something unexpected turns up and makes you stop, listen and most importantly, think. This was the headline from an American Association of Clinical Research (AACR) news release that I received from the San Antonio Breast Cancer Meeting (SABCS) this afternoon.
The result is totally unexpected. Why?
Well, we know the drug is effective in Stage IV metastatic disease and a previous trial from the Austrian group (study ABCSG XII) had positive results in stage I pre-menopausal women with breast cancer (see reference below). Early last year that trial was hailed as an important landmark study in The New York Times.
The current trial (AZURE) with zoledronic acid (Zometa) as adjuvant therapy in women stage II/III breast cancer was therefore expected to be positive, sitting neatly in the middle of the two previously published and positive settings.
Let’s take a look at the trial design.
3,360 women from several countries (the majority from the UK, Eire, Australia and Spain) were randomised to receive standard chemotherapy (physician’s choice), with one group receiving zoledronic acid and the other not. The women either had node positive (N+) adjuvant or T3/T4 or confirmed N+ neoadjuvant disease. They had undergone tumour resection, but not received bisphosphonates over the previous year or had any evidence of metastases. In the zoledronic acid arm, it was given as a 4mg infusion over a total of 5 years – ie 6 doses every 3-4 weeks with chemotherapy, then 8 doses every 3 months followed by maintenance therapy every 6 months for 5 cycles.
The primary endpoint of the study was disease free survival (DFS) with invasive DFS (IDFS) as a secondary endpoint. According to my notes, the planned final analysis was due to take place at 940 DFS events with an anticipated 3 year DFS of 75%, allowing for a 3 year recruitment period and 6 years of follow-up. The study was powered at 80% (two sided) to detect a 17% hazard reduction in DFS (HR=0.83) i.e. from 75% to 78.7% for DFS at 3 years. There was an original interim analysis in 2008 but the Data Monitoring Committee (DMC) did not recommend stopping the trial at that point. A second analysis was proposed (accepted) to the DMC given the low event rate for the whole study with a 3-year DFS rate of approximately 85%. The patients in both arms were well balanced for the key factors (lymph nodes, T stage, ER and menopausal status):
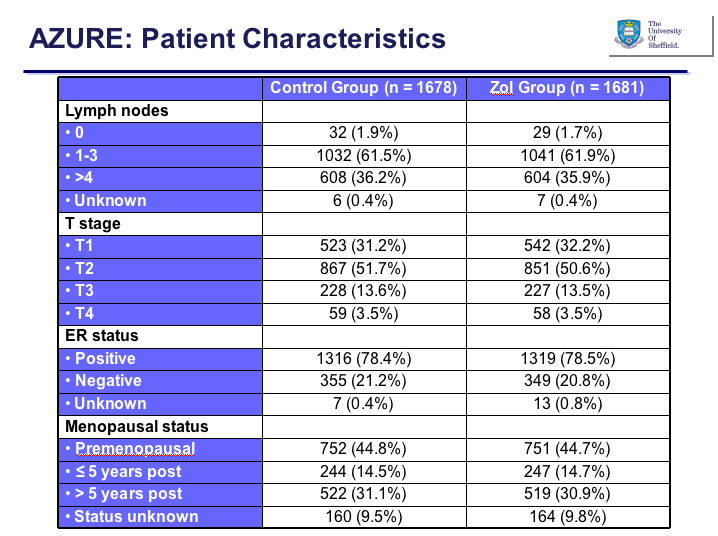
The groups were also well balanced for prior treatment, including chemotherapy:
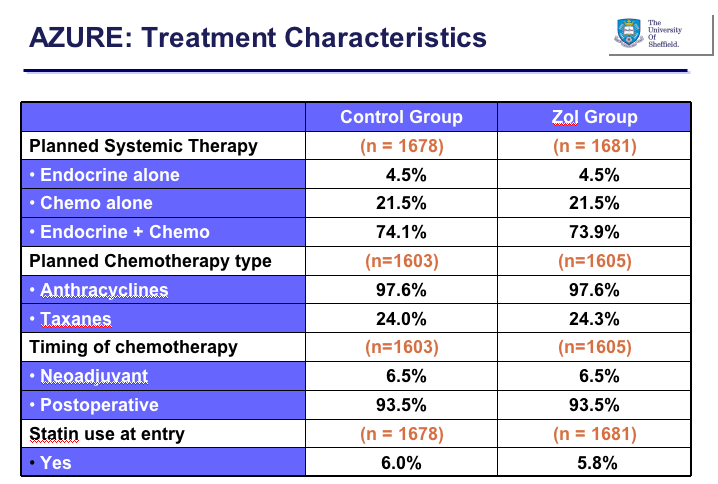
The median follow-up period was similar in both groups (58-60 months), as was time since last follow-up (approx. 6 months). There was little difference between the groups in adverse events, with the exception of osteonecrosis of the jaw (ONJ), a known side effect of bisphosphonate treatment:
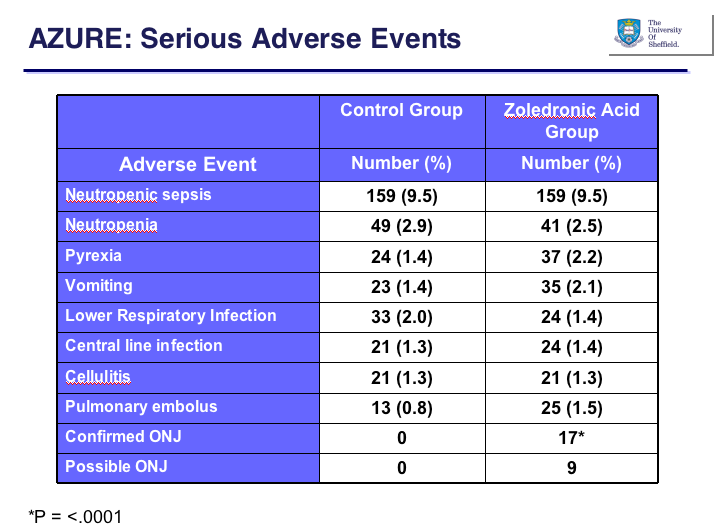
At this point, things started to get interesting. Here’s what happened in the ABCSG XII trial from Gnant et al.; you can see the clear separation of the DFS survival in their large (n=1803 ) trial in premenopausal women:
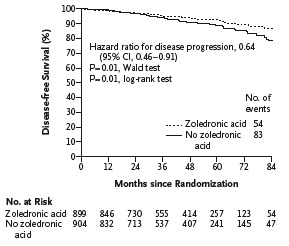
This is exactly what the investigators hoped to see with zoledronic acid in the AZURE trial. Instead, the survival curves virtually surprisingly overlap:
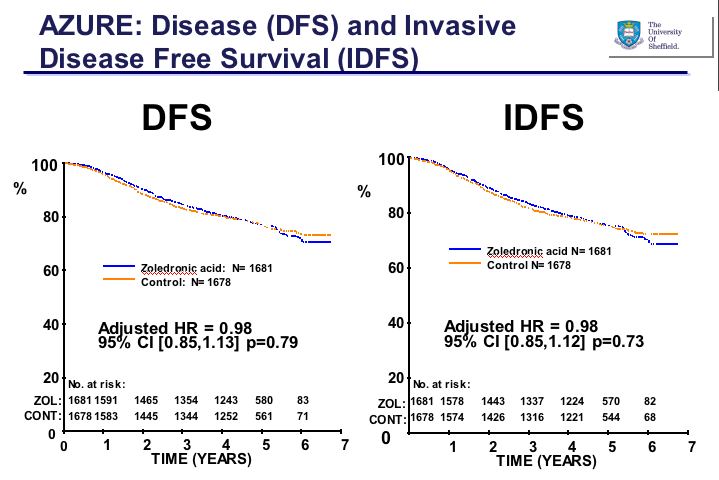
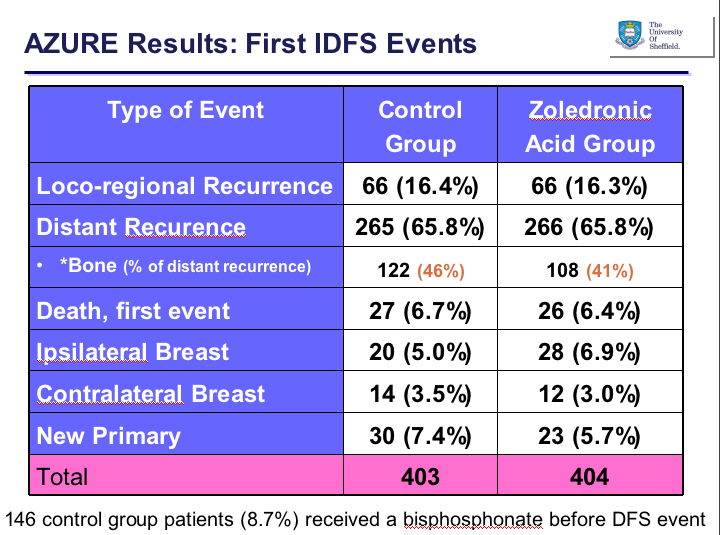
Just in case anyone is wondering whether prior bisphosphonate therapy affected the results, the analysis suggested otherwise:
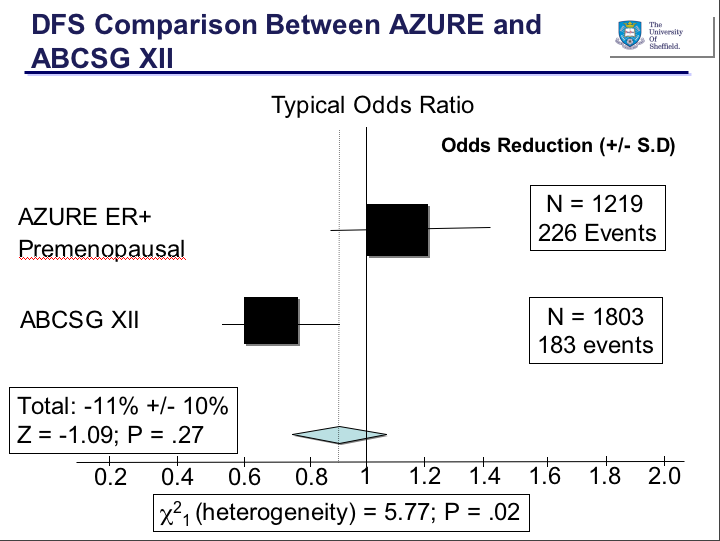
As Rob Coleman, the British PI of AZURE stated in the AACR press conference, the results between the two studies are essentially ‘chalk and cheese’, meaning polar opposites:
When they looked at the AZURE subgroup analysis, they found something interesting. While the premenopausal patients saw no benefit from zoledronic acid therapy, there was a significant difference for older women (>60yo) who were postmenopausal:
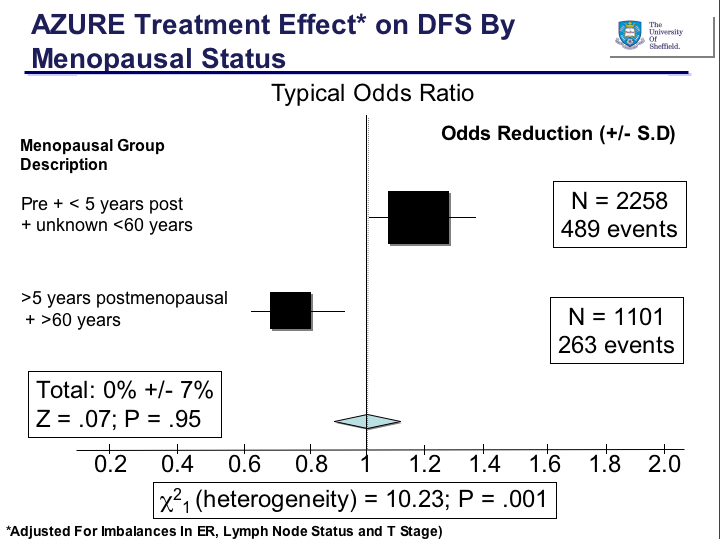
Given the disparity between the ABCSG XII and AZURE trials in premenopausal women, it is hard to imagine bone studies essentially being right, creating an interesting controversy as to why there is a disparity. Bear in mind that the Austrian study was in women with stage I breast cancer without chemotherapy, while AZURE was stage II/III disease with chemotherapy. Zoledronic acid in the Austrian trial was given at the same dose (4mg), but on a much simpler schedule (every 6 months) because there was no chemotherapy involved, only endocrine treatment.
Clearly something interesting is going on in the tumour microenvironment rather than the host and further research is likely to be ongoing to try and explain the science behind these findings.
Coleman speculated that there might be increased turnover and endocrine changes going on associated with menopausal and bone cell function, but that is just a hypothesis at this stage. My initial reaction is this does not explain the ABCSG XII results in stage I premenopausal women.
Impact of the AZURE results:
Given the resoundingly overall negative outcome in DFS, it is likely that Novartis will withdraw the expanded Zometa approval to prevent breast cancer relapse. Meanwhile, there will be much controversy and discussion to try and reconcile the results of the AZURE vs ABCSG XII trials in the premenopausal setting.
I think that it is unlikely that the guidelines will support routine use of zoledronic acid in this setting, although the findings in postmenopausal women will no doubt deserve further analysis in the coming months.
Disclosure:
I’m a former employee of Novartis and also a consultant, but have never worked on Zometa.
{UPDATE}
Novartis have announced that they are indeed with drawing the filing for Zometa, with the following statement:
“Last year, Novartis filed supplemental marketing authorization applications for the adjuvant treatment of premenopausal women with HR+ early breast cancer in conjunction with hormonal therapy in the US and European Union (EU) based on the results of ABSCG-12. Novartis is currently reviewing the data from the AZURE trial results, which were expected to be added to the submission. In the meantime, Novartis will withdraw the current marketing applications and discuss next steps with health authorities.”
References:
![]() Gnant, M., Mlineritsch, B., Schippinger, W., Luschin-Ebengreuth, G., Pöstlberger, S., Menzel, C., Jakesz, R., Seifert, M., Hubalek, M., Bjelic-Radisic, V., Samonigg, H., Tausch, C., Eidtmann, H., Steger, G., Kwasny, W., Dubsky, P., Fridrik, M., Fitzal, F., Stierer, M., Rücklinger, E., & Greil, R. (2009). Endocrine Therapy plus Zoledronic Acid in Premenopausal Breast Cancer New England Journal of Medicine, 360 (7), 679-691 DOI: 10.1056/NEJMoa0806285
Gnant, M., Mlineritsch, B., Schippinger, W., Luschin-Ebengreuth, G., Pöstlberger, S., Menzel, C., Jakesz, R., Seifert, M., Hubalek, M., Bjelic-Radisic, V., Samonigg, H., Tausch, C., Eidtmann, H., Steger, G., Kwasny, W., Dubsky, P., Fridrik, M., Fitzal, F., Stierer, M., Rücklinger, E., & Greil, R. (2009). Endocrine Therapy plus Zoledronic Acid in Premenopausal Breast Cancer New England Journal of Medicine, 360 (7), 679-691 DOI: 10.1056/NEJMoa0806285
2 Responses to “Zoledronic acid did not improve disease free survival in early breast cancer”
[…] Pharma Strategy Blog Commentary and insights on Pharma & Biotech new product development with a focus on oncology and hematology Skip to content HomeAboutConference ScheduleNewsletterWork with Us ← Zoledronic acid did not improve disease free survival in early breast cancer […]
Renal cancer and metastasis at bones, Zometa helps on the bone tumour but it made a maxilar necrosis that it is impossible to cure with a lot of pain (under oxycontin) ,posphates remains during 12 years in the body. Actually recurrence of the cancer and necrosis.
Comments are closed.