Using Flipboard to keep up with science and cancer research
A lot of people have asked me over the last year how I keep up with so much information in cancer research. I thought it would be a nice idea to illustrate one way I consume information on a daily basis.
Since getting an iPad2, my life has changed for the better. There are a number of really useful apps that let you browse information in a more user-friendly way. Four of these include:
- Zite
- Reeder
- Feedly
After trying them all over time, I found that for me, the one that resonated most for me was Flipboard.
What you get starts out like this:

Oh dear, that wasn’t welcome news this morning, bearing in mind I’m flying to ASCO in Chicago and then to London for the European Hematology Association meeting back to back in a few weeks. The return of #ashcloud tweets on Twitter looks imminent!
Here’s how Flipboard works…
- Select your Twitter lists
- Other people’s curated Twitter lists you follow
- RSS feeds for blogs, journals, pubmed searches, etc
And the inside of the magazine becomes organised into recognisable categories:
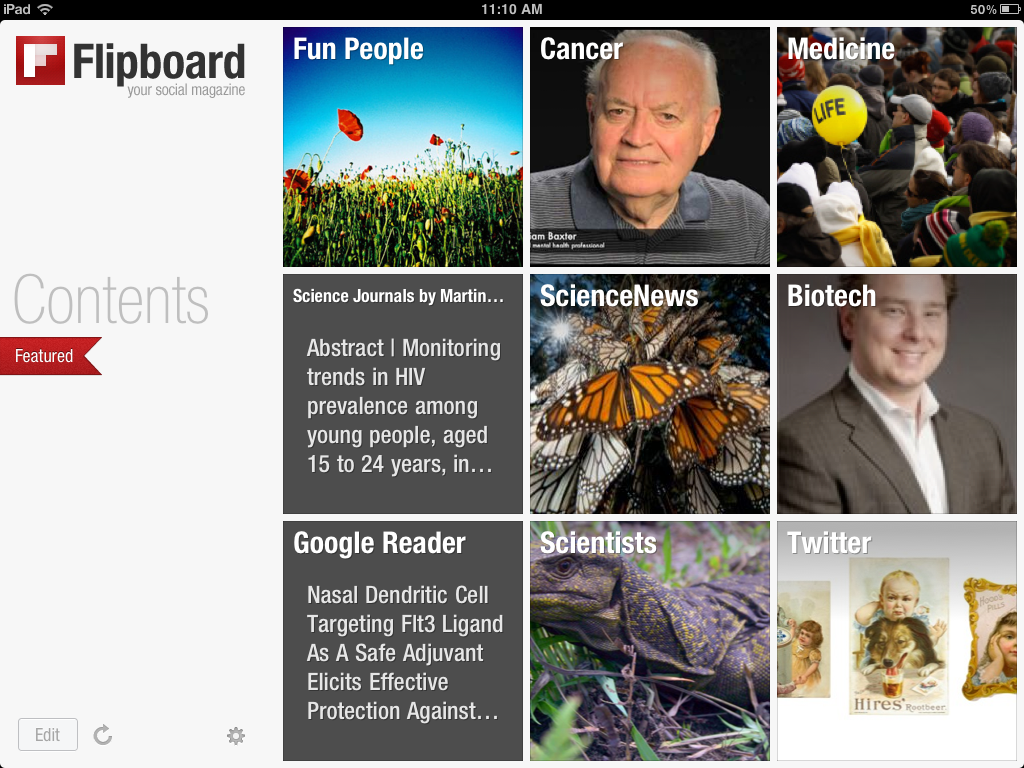
You can then flip through the categories and see what’s interesting to you for further reading. It also enables you to see broad trends much more easily.
Here’s an example from my journal and Pubmed feeds, since I have searches for all the key pathways that are associated with cancer. Some are more active than others, but over time, you get a mix of new articles whenever you browse them.
This is much easier to browse than reading lists and lists of things in Google Reader – Flipboard brings them life:

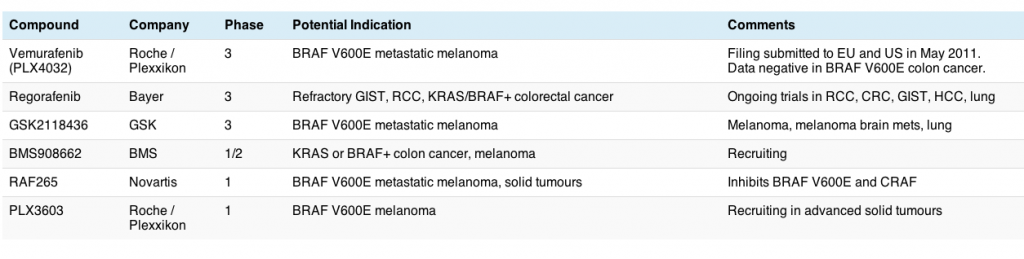
Now you can sort the chaff from the wheat – we know that the BRAF V600E mutation is important in melanoma, but not colon cancer for example, but I sure didn’t know it might have a role to play in thyroid cancer!
Reading the abstracts this way is much more impactful and user-friendly.
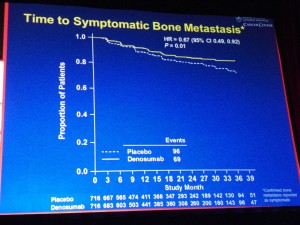
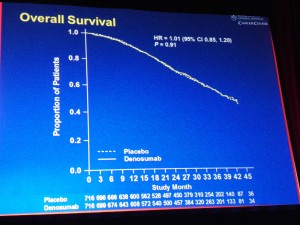
Another thing that is useful is browsing one’s Twitter lists on topics such as Cancer, Medicine etc. In the latter, I spotted an interesting tweet about how an app could be used to record your ECG on an iPhone – how cool is that?
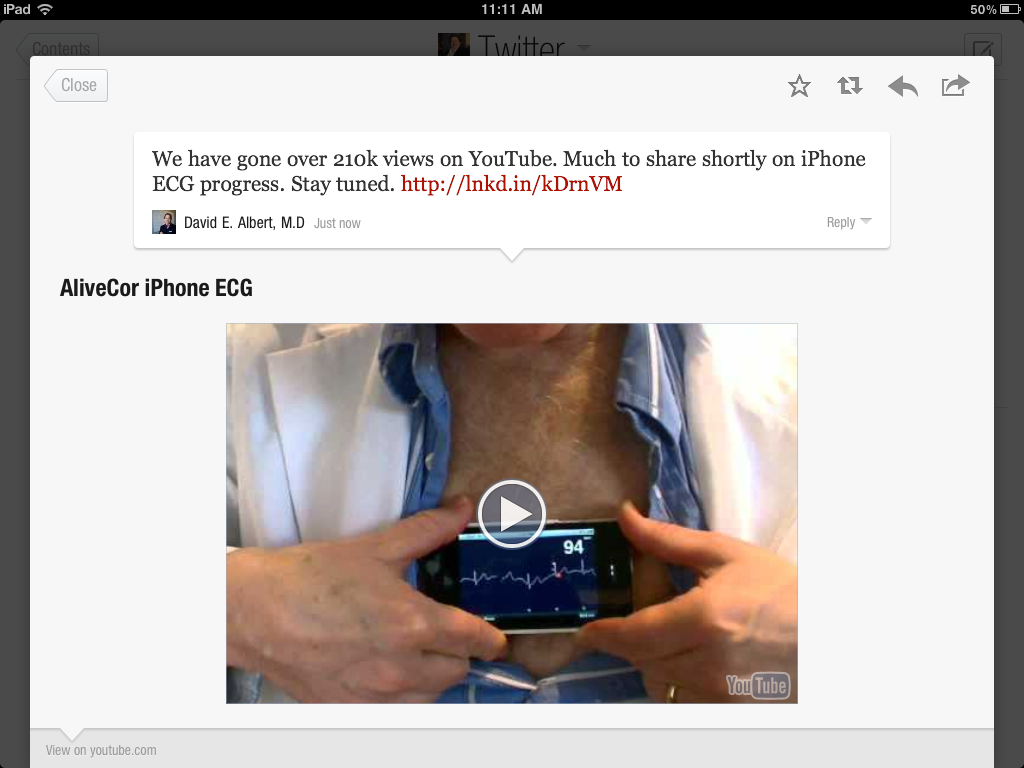
You can see the play button for the video on the iPad (it obviously won’t work on the photo though, in case you just clicked on it ;)) – you just click and listen while travelling or sitting in the comfort of your office.
The way technology has evolved over the last couple of years is simply amazing, but best of all, it makes processing information you have selected as relevant to your personal interests much more user-friendly and digestible.
That’s a big win for busy people on the go!
What apps do you like? Do share them in the comments below.
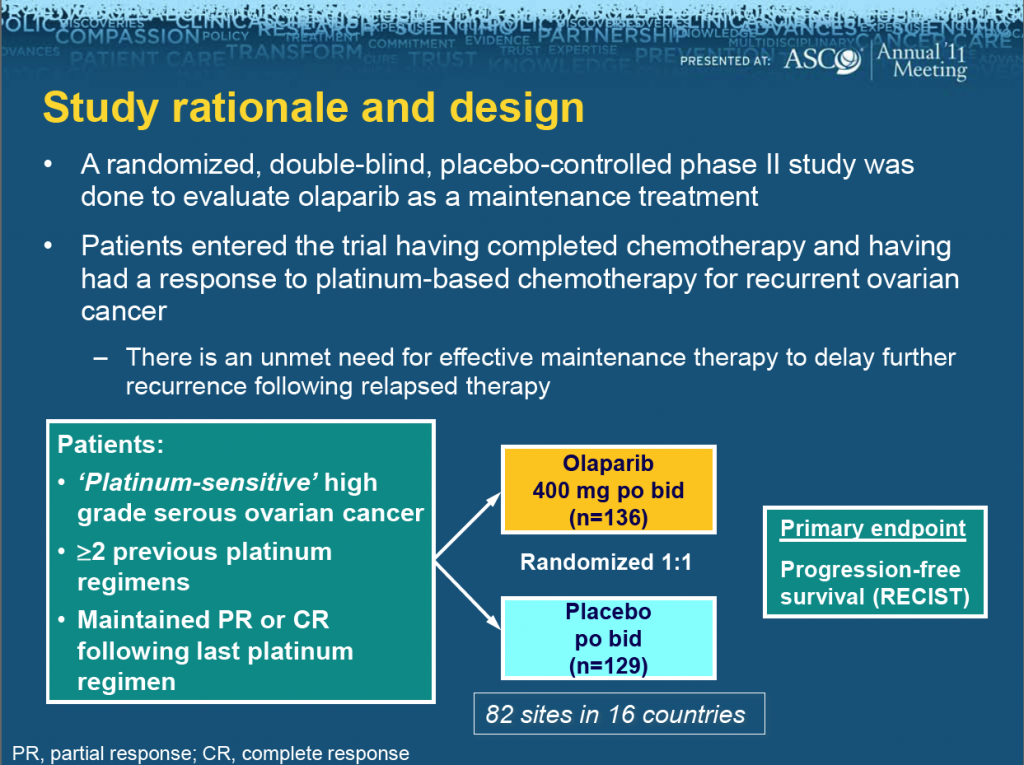
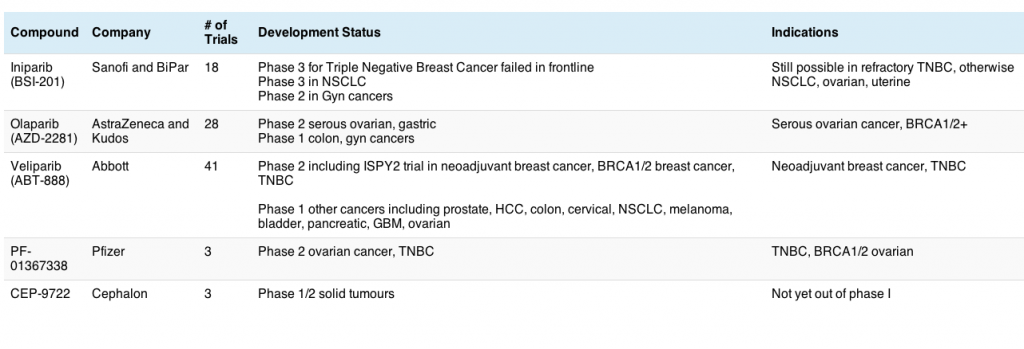
 ASCO president, Dr George Sledge of Indiana, announced that the meeting theme for this year is “Patients, Pathways, Progress” to reflect the growing focus on molecular targets to identify and treat patients more effectively.
ASCO president, Dr George Sledge of Indiana, announced that the meeting theme for this year is “Patients, Pathways, Progress” to reflect the growing focus on molecular targets to identify and treat patients more effectively.