New mechanisms of resistance to MET inhibition
A very apt quote from Jeff Engelman’s group caught my eye this week:
“Unfortunately, cancers invariably develop resistance, and overcoming or preventing resistance will ultimately be key to unleashing their full therapeutic potential.”
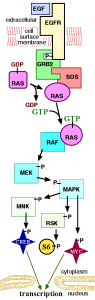
MET is the receptor tyrosine kinase for hepatocyte growth factors (HGF) and inhibition has been implicated in metastases and migration of cancer cells (Rong et al., (1994), Takayama et al., (1997)), but more recently, it has also been observed that some tumour types have MET oncogenic addiction, including gastric cancer (Smolen et al., 2006).
Qi et al., (2011) went on to explain how they were looking at strategies for overcoming resistance to MET inhibitors, using PHA-665752 and PF-2341066, as an example in highly sensitive gastric cell lines. They investigated the possibilities in vivo and in vitro. The results, however, were unexpected:
“To our surprise, we observed at least two mechanisms of resistance that arose simultaneously. Both resulted in maintenance of downstream PI3K (phosphoinositide 3-kinase)-AKT and MEK (MAP/ERK kinase)-ERK signaling in the presence of inhibitor.”
Many of you will be aware of activation loops from other kinases, such as imatinib (Gleevec) in CML (T315I) and GIST (D842V) or erlotinib (Tarceva) in lung cancer (T790M), and adaptive pathways e.g. with BRAF inhibitors such as PLX4032 (vemurafenib) in melanoma, so this phenomenon is not uncommon.
With the MET inhibitors tested in the current research, the group found:
- A mutation in the MET activation loop, Y1230
- Activation of the epidermal growth factor receptor (EGFR) pathway due to increased expression of transforming growth factor alpha (TGFa)
What do these results mean?
The data suggests that combining MET and EGFR inhibitors in gastric cancer may be a viable therapeutic strategy, but consideration must also be given to approaches that inhibit Y1230 mutant MET as well, in order to shut off the escape routes.
It is hard to argue with the authors conclusion that:
“These results also underscore the notion that a single cancer can simultaneously develop resistance induced by several mechanisms and highlight the daunting challenges associated with preventing or overcoming resistance.”
Given the positive results seen with trastuzumab (Herceptin) in patients with HER2-positive gastric cancer, part of me is also wondering what incremental value there would be efficacy-wise, if MET and EGFR inhibitors were used in combination with trastuzumab? We know that the blocking the driver mutation, the adaptive pathway and the ligand is important. Some further preclinical research in this area may shed light on the matter.
References:
![]() Qi, J., McTigue, M., Rogers, A., Lifshits, E., Christensen, J., Janne, P., & Engelman, J. (2011). Multiple Mutations and Bypass Mechanisms Can Contribute to Development of Acquired Resistance to MET Inhibitors Cancer Research, 71 (3), 1081-1091 DOI: 10.1158/0008-5472.CAN-10-1623
Qi, J., McTigue, M., Rogers, A., Lifshits, E., Christensen, J., Janne, P., & Engelman, J. (2011). Multiple Mutations and Bypass Mechanisms Can Contribute to Development of Acquired Resistance to MET Inhibitors Cancer Research, 71 (3), 1081-1091 DOI: 10.1158/0008-5472.CAN-10-1623
Ma, P., Tretiakova, M., Nallasura, V., Jagadeeswaran, R., Husain, A., & Salgia, R. (2007). Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion British Journal of Cancer, 97 (3), 368-377 DOI: 10.1038/sj.bjc.6603884
Rong S, Segal S, Anver M, Resau JH, & Vande Woude GF (1994). Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proceedings of the National Academy of Sciences of the United States of America, 91 (11), 4731-5 PMID: 8197126
Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, & Merlino G (1997). Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proceedings of the National Academy of Sciences of the United States of America, 94 (2), 701-6 PMID: 9012848
Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC, Christensen JG, Settleman J, & Haber DA (2006). Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proceedings of the National Academy of Sciences of the United States of America, 103 (7), 2316-21 PMID: 16461907


 Maybe two feet under snow really, judging by my back yard, which is largely sheltered and not prone to drifts. It looks like a pristine Winter Wonderland.
Maybe two feet under snow really, judging by my back yard, which is largely sheltered and not prone to drifts. It looks like a pristine Winter Wonderland.