Highlights and lowlights from the ASH meeting #ASH2010
Since the pre-ASH review of what might be hot at the conference, a few people have written to me asking for a rundown on my highlights from the recent hematology and breast cancer conferences. In the end, I only made it ASH in person, but followed the tweets and news that flowed out of the AACR SABCS meeting, which will post either later today or tomorrow.
Here are a few observations that struck me from ASH. There were some genuine highlights from this meeting, both in lymphomas and leukemias:
Rituximab (Roche) was the only clinical abstract selected for the plenary session this year. I loved Dr Anas Younes’ summary of this data on his Facebook Page:
“Clearly, early treatment with rituximab improved the progression free survival and time to next therapy. It is too early to see any effect on survival. My guess is that watch and wait will disappear as patients and doctors will start recommending early use of rituximab instead of watch and worry!”
The results are therefore potentially practice changing and it’s good to see positive data in a difficult to treat subset.
Ponatinib (Ariad), a third generation tyrosine kinase inhibitor (TKI) had excellent phase I data in relapsed/refractory chronic myeloid leukemia (CML), both with and without the T315I mutation. The phase II trial (PACE) is now enrolling and it is my hope that patients who find the existing TKIs fail or develop the T315I mutation will have a new therapeutic option in the not too distant future if the results continue to hold up.
Brentuximab vedotoxin (Seattle Genetics and Millennium) was the real star at ASH this year though. We’ve covered some background on the blog here. Basically, it’s a novel conjugate consisting of a monoclonal antibody with a chemotherapy molecule bolted on. The data in Hodgkin Lymphoma (HL) was absolutely stunning and a nice follow up to the article published in the NEJM by Dr Anas Younes et al., earlier this year. The companies have since announced that they intend to file with the FDA in early 2011, so perhaps we might see this exciting agent approved in the second half of next year.
Unfortunately, there were also some disappointing lowlights:
Lenalidomide (Celgene) had some interesting data in several phases of multiple myeloma, but what shocked many observers was the results of the MM-015 trial. This was the third study to suggest that there may be a risk for secondary cancers with Revlimid. Previously, the data emanated from the IFM and CALGB cooperative studies, but this was the first company sponsored trial that may be considered a registration study.
Clearly, the trend is not good news at all, although it is known that:
- Alkylating treatment for AML and NHL run the risk of secondary cancers later in life
- The literature suggests that all three cancers have a higher risk of secondary cancers developing, which may or may not be drug related.
In the light of all this, the myeloma finding with Revlimid is not entirely surprising but one can see why investors panicked and sold off stock after the data was announced. Of note though is that patients in the maintenance arm (MPR-R), however, will clearly receive more exposure to the drug than those in the MPR arm, potentially exposing them to a slightly higher risk.
Bosutinib (Pfizer) is a 2nd generation TKI in phase III development for newly diagnosed CML. Much of the expectation surrounding this agent was whether is would be superior to imatinib (Gleevec) in terms of efficacy in the same way that nilotinib (Novartis) and dasatinib (BMS). Both have proven to deliver earlier and deeper responses than imatinib, albeit with the caveat that we won’t know whether survival and overall outcomes will be better until the 5-10 year data is available. There was also some suggestion from Pfizer evolving over the last year that bosutinib might also be cleaner given that it only inhibits BCR-ABL and Src and not other off-target kinases such as PDGF. Unfortunately, that turned out to be a complete misnomer.
Dr Carlos Gambacorti-Passerini (Milan) did an excellent job trying to put a positive spin on the data, but this was a resoundingly negative trial. The primary endpoint of confirmed complete cytogenetic response (CCyR) at 12 months in the intent to treat (ITT) population was not met, although the secondary endpoint of major molecular response (MMR) was met. The side effect profile was particularly disappointing with a lot of diarrhoea, nausea and vomiting being frequent and most unwelcome side effects, which are very similar to chemotherapy. Much of the goal of targeted therapy is to hit the cancer cells for six while leaving normal cells largely alone. Chemotherapy is highly untargeted and hits everything in it’s wake, leaving people well aware that they are undergoing treatment for cancer rather than living with a chronic condition. Sometimes a disconnect between scientific theory and reality happens.
Pfizer announced at the meeting that they plan on pursuing an EU filing, but would hold discussions with the FDA about the relapsed/refractory setting, suggesting they will not file in the US for newly diagnosed CML, unless the FDA do a U-turn on the secondary (MMR) endpoint. The chances of that are rather slim, I suspect. I’m not entirely sure how the filing might be suitable for the EMA and not the FDA, unless the primary endpoints agreed with the Health Authorities were different. That said, given the good choices already available to people with CML, bosutinib is not going to appeal to many, I’m afraid. Certainly the CML KOLs I spoke to at the meeting were largely unenthusiastic about the agent.
Like many of the attendees, the thing most remembered from Orlando will be the bitter cold, both outside and in the presentation halls! Overall, it was really a rather quiet meeting this year, except for the excellent data in brentuximab.
 After the recent cancer conference and travel schedule, I’m a bit pushed for time today as the consulting work we’re doing at Icarus is frantically busy at the moment.
After the recent cancer conference and travel schedule, I’m a bit pushed for time today as the consulting work we’re doing at Icarus is frantically busy at the moment.
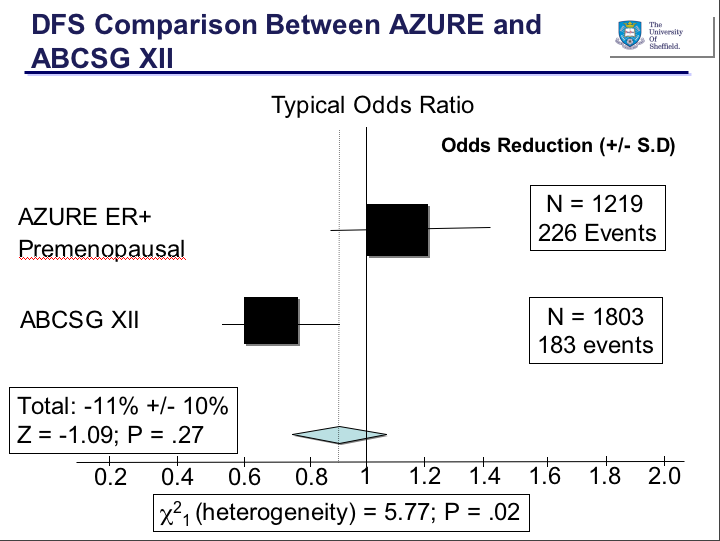
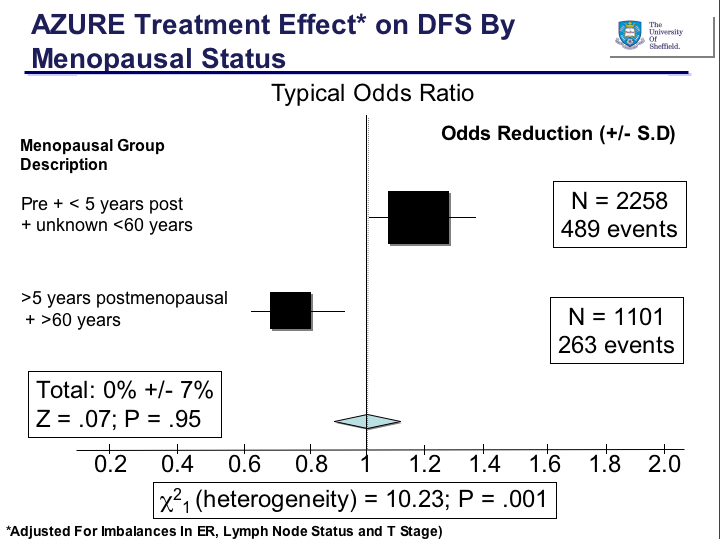
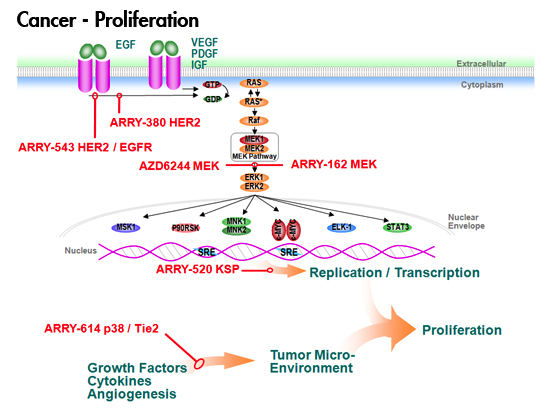
 This year, there was no new data or ideas expounded in the sessions I chose, they were basically a run through of the old data with a couple of slides at the end listing a few compounds that are in development. The pipeline in for hematologic malignancies is gradually gaining more attention from Pharma, which is great news, although they are probably a lot less crowded than the main solid tumours.
This year, there was no new data or ideas expounded in the sessions I chose, they were basically a run through of the old data with a couple of slides at the end listing a few compounds that are in development. The pipeline in for hematologic malignancies is gradually gaining more attention from Pharma, which is great news, although they are probably a lot less crowded than the main solid tumours.
 Later in the poster session, I tracked down Michael Becker (
Later in the poster session, I tracked down Michael Becker ( If you’re not watching Semafore and another PI3-kinase company, Calistoga, that I mentioned in my What’s Hot at ASH 2010 post, then check them out as they both have novel and interesting compounds that may be eventually licensed by pharma or biotech companies. You can also read more about the background behind PI3-kinase as a valid cancer target
If you’re not watching Semafore and another PI3-kinase company, Calistoga, that I mentioned in my What’s Hot at ASH 2010 post, then check them out as they both have novel and interesting compounds that may be eventually licensed by pharma or biotech companies. You can also read more about the background behind PI3-kinase as a valid cancer target