BRAF mutations in hairy cell leukemia
Last month an interesting article was published in The New England Journal of Medicine describing how BRAFV600E mutations may have a key role to play in hairy cell leukemia (HCL), which came out around the same time as the European Hematology Association (EHA) meeting that I attended in London. The news certainly caused a buzz at the conference!
Source: Wikipedia
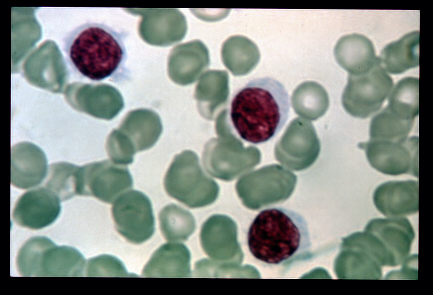
Hair cell leukemia is a fairly rare type of leukemia that affects B cells (lymphocytes), which are distinguished by their hairy like appearance under the microscope because they have fine projections coming from their surface.
Over the past year, we have heard much about how the BRAFV600E mutation plays a critical role in melanoma and the progress with the testing of a specific inhibitor, vemurafenib (PLX4032), in the clinic, leading to some initial clinical success in this indication. What’s particularly interesting about the NEJM article is that it describes, for the first time, how the BRAFV600E mutation may be a key genetic alteration in HCL.
The researchers used Sanger sequencing to undertake an extensive analysis of the genome in normal and HCL peripheral blood samples. The findings were also validated in additional patients with HCL (n=47). The results were a little surprising:
“Whole-exome sequencing identified five missense somatic clonal mutations that were confirmed on Sanger sequencing, including a heterozygous mutation in BRAF that results in the BRAF V600E variant protein.”
The mutation was only found in patient samples who had HCL, not other types of leukemia or lymphomas:
“None of the 195 patients with other peripheral B-cell lymphomas or leukemias who were evaluated carried the BRAF V600E variant, including 38 patients with splenic marginal-zone lymphomas or unclassifiable splenic lymphomas or leukemias.”
Some immunohistologic and Western blot studies, were performed. They found that:
“HCL cells expressed phosphorylated MEK and ERK (the downstream targets of the BRAF kinase), indicating a constitutive activation of the RAF–MEK–ERK mitogen-activated protein kinase pathway in HCL.
In vitro incubation of BRAF-mutated primary leukemic hairy cells from 5 patients with PLX-4720, a specific inhibitor of active BRAF, led to a marked decrease in phosphorylated ERK and MEK.”
PLX-4720 is another BRAF inhibitor that Plexxikon have in development in addition to the original one, PLX-4032 that became vemurafenib.
Now, while is promising evidence that needs to be researched further, we must exercise caution. Remember that just because a mutation exists, does not mean that it is a key driver. We saw this with colon cancer and BRAFV600E mutations – where vemurafenib had little of no effect in patients, despite promising preclinical data – a stark contrast to the results in metastatic melanoma! Why does the same target produce entirely different results when inhibited by an effective agent? One reason could be that that BRAF is a passenger not a driver in colon cancer.
In the meantime, I will be keenly following any progress with testing of specific BRAF inhibitors for patients with hairy cell leukemia to see whether it will be a useful clinical approach in managing the disease or not.
References:
![]() Tiacci, E., Trifonov, V., Schiavoni, G., Holmes, A., Kern, W., Martelli, M., Pucciarini, A., Bigerna, B., Pacini, R., Wells, V., Sportoletti, P., Pettirossi, V., Mannucci, R., Elliott, O., Liso, A., Ambrosetti, A., Pulsoni, A., Forconi, F., Trentin, L., Semenzato, G., Inghirami, G., Capponi, M., Di Raimondo, F., Patti, C., Arcaini, L., Musto, P., Pileri, S., Haferlach, C., Schnittger, S., Pizzolo, G., Foà, R., Farinelli, L., Haferlach, T., Pasqualucci, L., Rabadan, R., & Falini, B. (2011). Mutations in Hairy-Cell Leukemia New England Journal of Medicine, 364 (24), 2305-2315 DOI: 10.1056/NEJMoa1014209
Tiacci, E., Trifonov, V., Schiavoni, G., Holmes, A., Kern, W., Martelli, M., Pucciarini, A., Bigerna, B., Pacini, R., Wells, V., Sportoletti, P., Pettirossi, V., Mannucci, R., Elliott, O., Liso, A., Ambrosetti, A., Pulsoni, A., Forconi, F., Trentin, L., Semenzato, G., Inghirami, G., Capponi, M., Di Raimondo, F., Patti, C., Arcaini, L., Musto, P., Pileri, S., Haferlach, C., Schnittger, S., Pizzolo, G., Foà, R., Farinelli, L., Haferlach, T., Pasqualucci, L., Rabadan, R., & Falini, B. (2011). Mutations in Hairy-Cell Leukemia New England Journal of Medicine, 364 (24), 2305-2315 DOI: 10.1056/NEJMoa1014209
 Today several people have reminded me that it’s Canada Day and also the Independence Day weekend in the US. Although I’m British and celebrate neither, there will be a short hiatus from blogging in honour of my Canuck and American friends.
Today several people have reminded me that it’s Canada Day and also the Independence Day weekend in the US. Although I’m British and celebrate neither, there will be a short hiatus from blogging in honour of my Canuck and American friends.

 This morning I was pondering a triangulation of several random thoughts that appeared in my Twitter stream, many from BIO, about various topics:
This morning I was pondering a triangulation of several random thoughts that appeared in my Twitter stream, many from BIO, about various topics: