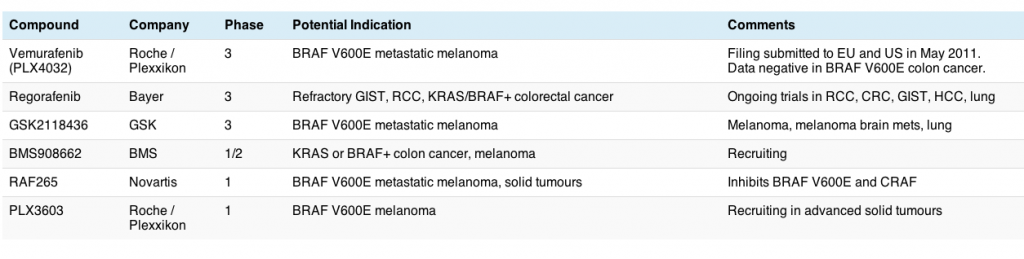
Advanced prostate cancer has been quite a hot topic lately, with several new and relatively late stage compounds in the pipeline garnering attention from promising data. One of those agents, abiraterone acetate (Zytiga) only just received FDA approval on Friday and has been designated for accelerated review by the EMEA.
Following on from previous interviews in the Pharma Strategy Blog “Making a Difference” series with Dr Sue Desmond-Hellman (Chancellor of the University of California, San Francisco), Alain Moussy (CEO of AB Sciences) and Dr Ross Camidge (University of Colorado), it seemed most timely to extend the next round of the series to prostate cancer.
It was therefore a pleasure to talk with Dr Sawyers about his current research in the prostate cancer last week and discuss how he approaches some of the challenges involved with incorporating translational medicine into clinical research. He is co-inventor of two drugs currently in clinical trials for prostate cancer, namely MDV3100 (Medivation) and ARN-509 (Aragon Pharmaceuticals).

Charles L. Sawyers, MD is Chair of the Human Oncology and Pathogenesis Program at the Memorial Sloan-Kettering Cancer Center (MSKCC), and an Investigator with the Howard Hughes Medical Institute. In 2009, he received the Lasker-Debakey Clinical Medical Research Award along with Drs Brian Druker and Nick Lydon, for their work on molecular targeting that led to the development of imatinib (Gleevec/Glivec), a drug that revolutionized the treatment of Chronic Myeloid Leukemia (CML) and turned it from deadly cancer into a manageable, chronic disease.
In full disclosure, I had the great privilege of working with Drs Sawyers and Druker while bringing imatinib to market at Novartis Oncology.
Pharma Strategy Blog: Charles, you and I have known each other for over ten years, when we first met you were at UCLA. What made you move to the East Coast and MSKCC?
Dr Sawyers: Harold Varmus who was the Director here at MSKCC, before he moved to the NCI, made me a job offer I couldn’t refuse. Memorial had built up an impressive cadre of basic scientists, but there was this missing piece of physician scientists who could capitalize on translational opportunities. He was able to convince “the powers that be” to build a new research tower with 21 floors of lab space, that opened in 2006. He offered me 3 floors and the opportunity to be Director of a brand new program called “Human Oncology.”
My mission was to recruit the best and brightest physician scientists either locally or around the country. I also saw, after my imatinib work, that the most important contributions I could continue to make from my laboratory work were not going to be in CML, and I wanted a new challenge. I had started to work on prostate cancer for many reasons, mostly scientific, and I needed to be at a place where clinical care and clinical trials infrastructure was much more integrated than it was at UCLA. So, it was not that hard a decision to make the move.
Pharma Strategy Blog: One of the drugs that you discovered at your lab was MDV3100, what are you thoughts on when this may be used?
Dr Sawyers: I am very much involved in asking translational questions about MDV3100 and whether it works beyond castrate resistant disease. Does it work up front in the neo-adjuvant setting, prior to surgery to shrink the tumor? Would it synergize with radiation? All kinds of interesting questions are coming up that we are working to answer.
Pharma Strategy Blog: Why does MDV3100 block the androgen receptor better than bicalutamide?
Dr Sawyers: The most interesting property that MDV3100 has, and what I think is the most likely explanation for its superior performance, is that when you treat cells with this compound the androgen receptor is completely incapable of binding DNA. We have shown this recently using ChIP-Seq technology that is very powerful at annotating all the binding sites for any transcription factor across the genome. With bicalutamide, the androgen receptor still binds with the drug very tightly on many thousands of binding sites, whereas with MDV3100, we cannot find it binding anywhere. It has a profoundly different effect on the receptor.
Pharma Strategy Blog: How did the discovery of MDV3100 come about?
Dr Sawyers: We had been using mouse models to understand why the tumor became resistant to castration and bicalutamide. What came out of that was the level of expression of the androgen receptor was consistently up, about 3 to 5 fold, in the castrate resistant sub lines of otherwise sensitive tumors. Then we showed by either over-expressing the androgen receptor at about that level or knocking it down in castrate resistant lines, that it was both necessary and sufficient for this resistance phenotype. Quite dramatically, when you overexpress the receptor at that level and treat cells with bicalutamide, bicalutamide is now a weak agonist rather than antagonist. So, you can trick the cell into responding differently just by manipulating the level of the androgen receptor.
All of that led me to approach a couple of companies that were interested in prostate cancer, with the idea that we should do a screen for compounds that are selected based on their ability to inhibit androgen receptor signaling in this context of higher expression. Everybody that I talked to in the pharma industry pretty much thought that the androgen receptor was not really all that relevant a target in castrate resistant disease. There seemed to be a mindset, that had built up over decades, that castrate resistant disease was really androgen independent disease, and therefore hormone therapy is no longer going to be effective.
That’s why we had to do it academically, and the approach that worked was based on a friendship that I had made with a chemist at UCLA named Mike Jung. Rather than do high-throughput screens, he said there’s tons of chemistry already done on the androgen receptor, let’s explore that literature and try to find compounds that bind with extremely high affinity that others have described that aren’t antagonists and then do some SAR to figure out how to make them antagonists. He found this compound that was described in an old patent that has extremely high binding affinity for the androgen receptor, never went anywhere because it is a potent agonist, but it was about two orders of magnitude tighter than bicalutamide. So he made it, we tested it and of course it didn’t work. Then we started making derivatives of that compound, tested 200 over a year and half, and stumbled upon MDV3100.
Pharma Strategy Blog: What is the current state of development for MDV3100?
Dr Sawyers: MDV3100 is now in a phase 3 registration trial that is fully accrued and is supposed to read out later this year, maybe early 2012.
Pharma Strategy Blog: What do you think of Circulating Tumor Cells (CTCs) as a surrogate marker in prostate cancer instead of PSA response?
Dr Sawyers: Measuring CTCs using a standard Veridex platform is very nice, the answer that is not so clear is whether a CTC drop is predictive of a long-term clinical benefit? There are a number of clinical trials in prostate cancer moving along with traditional survival endpoints in which the CTC data is being collected in parallel. Hopefully, over another a year or two these kind of correlates can be drawn to see if it is a surrogate marker of response that could lead to faster registration.
Pharma Strategy Blog: Could CTCs replace PSA as a measure of response?
Dr Sawyers: I think in the case of MDV3100 we are targeting the androgen receptor, which regulates the expression of PSA, so it is almost a given that if your drug is engaging the target effectively you have to see a PSA drop. If you don’t you probably haven’t hit the target correctly. In essence, PSA is a pharmacodynamic endpoint. If you are able to sustain PSA down for 12 weeks, with a drop of at least 50%, that is considered a pretty significant effect that is likely to be predictive of some other longer-term benefit. Not many drugs have done that in the past, so I wonder if PSA actually might be more valuable than we give it credit for, if we just set the bar higher for what we call a PSA response.
Pharma Strategy Blog: Can you tell us more about the other prostate cancer compound that came out of your lab that is being developed by Aragon?
Dr Sawyers: “Son of Medivation” is what some people call it. It came out later than MDV3011 and is more potent, and has what we think is a better safety profile. It is called ARN-509 and is in the clinic now. It is still in the dose-escalation stage of a phase I study at Sloan Kettering that Howard Scher and colleagues are running. There is a lot of excitement around it and we are pushing as fast as we can. The challenge now is that the prostate cancer space is becoming crowded.
Pharma Strategy Blog: Does ARN-509 have a similar mechanism of action to MDV3100?
Dr Sawyers: Yes, very similar. We don’t yet know if ARN-509 will work in those patients who don’t respond to MDV3100 or have resistance to it. If it does work in that setting in the clinic, then it is a straightforward path to approval. What I think is more likely is that ARN-509 will work in a similar same patient population as MDV3100 but might produce a higher percentage of responders or maybe longer duration of response. It will take at least a year if not a little more to know with confidence what those numbers are for ARN-509 compared to MDV3100, and by then Medivation will be approved.
Pharma Strategy Blog: How do androgen receptor antagonists such as MDV3100 and ARN-509 compare to abiraterone acetate (Zytiga) that was recently approved by the FDA?
Dr Sawyers: Abiraterone is targeting the androgen receptor pathway differently. Even though all these men remain on a testosterone lowering agent, testosterone is still produced primarily by the adrenal gland. Abiraterone targets the enzyme Cyp17 that is critical in maintaining that residual level of testosterone. It is the same target of ketoconzole, a drug that has been used in this space, but has a fairly unpleasant side-effect profile. Abiraterone is looking great and showed a survival advantage in the same kind of trial as the Medivation one. A very obvious question is whether it would make sense to target the androgen receptor pathway at two points i.e. abiraterone plus MDV3100. Scientifically it makes beautiful sense and I think that combination trials will happen.
Pharma Strategy Blog: Would it make sense to potentially sequence them?
Dr Sawyers: I am always a believer of going up front with your best shot, so scientifically favor using a combination.
Pharma Strategy Blog: What are some of the challenges that remain in prostate cancer?
Dr Sawyers: We have a good understanding of the prostate genome, but it is very challenging to obtain tissue from patients in trials so that we can subset them into molecular subgroups. The benefit of that is so crystal clear in other tumor types. It is a challenge that we are still struggling how to execute in prostate. One reason for this is that the trials are typically done with end-stage patients with bone disease, so tissue is not easily obtainable. Even if patients give consent, technically, it is a challenge to isolate the tumor and analyse it.
Pharma Strategy Blog: It is a very exiting time to be in this field. Hopefully, we will learn more at the AACR special meeting on Prostate Cancer that you are organizing in Orlando next year. Thank you, Dr Sawyers, for sharing your thoughts and insights.
References:
 Scher, H., & Sawyers, C. (2005). Biology of Progressive, Castration-Resistant Prostate Cancer: Directed Therapies Targeting the Androgen-Receptor Signaling Axis Journal of Clinical Oncology, 23 (32), 8253-8261 DOI: 10.1200/JCO.2005.03.4777
Scher, H., & Sawyers, C. (2005). Biology of Progressive, Castration-Resistant Prostate Cancer: Directed Therapies Targeting the Androgen-Receptor Signaling Axis Journal of Clinical Oncology, 23 (32), 8253-8261 DOI: 10.1200/JCO.2005.03.4777
Watson, P., Chen, Y., Balbas, M., Wongvipat, J., Socci, N., Viale, A., Kim, K., & Sawyers, C. (2010). Inaugural Article: Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor Proceedings of the National Academy of Sciences, 107 (39), 16759-16765 DOI: 10.1073/pnas.1012443107
 This morning I was pondering a triangulation of several random thoughts that appeared in my Twitter stream, many from BIO, about various topics:
This morning I was pondering a triangulation of several random thoughts that appeared in my Twitter stream, many from BIO, about various topics: